Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy
- PMID: 23757309
- PMCID: PMC3807652
- DOI: 10.1161/CIRCULATIONAHA.112.001075
Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy
Abstract
Background: Relevant preclinical models are necessary for further mechanistic and translational studies of c-kit+ cardiac stem cells (CSCs). The present study was undertaken to determine whether intracoronary CSCs are beneficial in a porcine model of chronic ischemic cardiomyopathy.
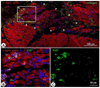
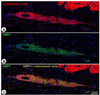
Methods and results: Pigs underwent a 90-minute coronary occlusion followed by reperfusion. Three months later, autologous CSCs (n=11) or vehicle (n=10) were infused into the infarct-related artery. At this time, all indices of left ventricular (LV) function were similar in control and CSC-treated pigs, indicating that the damage inflicted by the infarct in the 2 groups was similar; 1 month later, however, CSC-treated pigs exhibited significantly greater LV ejection fraction (echocardiography) (51.7±2.0% versus 42.9±2.3%, P<0.01), systolic thickening fraction in the infarcted LV wall, and maximum LV dP/dt, as well as lower LV end-diastolic pressure. Confocal microscopy showed clusters of small α-sarcomeric actin-positive cells expressing Ki67 in the scar of treated pigs, consistent with cardiac regeneration. The origin of these cycling myocytes from the injected cells was confirmed in 4 pigs that received enhanced green fluorescent protein -labeled CSCs, which were positive for the cardiac markers troponin I, troponin T, myosin heavy chain, and connexin-43. Some engrafted CSCs also formed vascular structures and expressed α-smooth muscle actin.
Conclusions: Intracoronary infusion of autologous CSCs improves regional and global LV function and promotes cardiac and vascular regeneration in pigs with old myocardial infarction (scar). The results mimic those recently reported in humans (Stem Cell Infusion in Patients with Ischemic CardiOmyopathy [SCIPIO] trial) and establish this porcine model of ischemic cardiomyopathy as a useful and clinically relevant model for studying CSCs.
Keywords: angiogenesis inducers; heart failure; muscle development; myocardial infarction; stem cells.
Conflict of interest statement
Figures










Comment in
-
Mechanisms of cell therapy for clinical investigations: an urgent need for large-animal models.Circulation. 2013 Jul 9;128(2):92-4. doi: 10.1161/CIRCULATIONAHA.113.003869. Epub 2013 Jun 11. Circulation. 2013. PMID: 23757310 Free PMC article. No abstract available.
References
-
- Farahmand P, Lai TY, Weisel RD, Fazel S, Yau T, Menasche P, Li RK. Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation. 2008;118:S130–S137. - PubMed
-
- Waksman R, Fournadjiev J, Baffour R, Pakala R, Hellinga D, Leborgne L, Yazdi H, Cheneau E, Wolfram R, Seabron R, Horton K, Kolodgie F, Virmani R, Rivera E. Transepicardial autologous bone marrow-derived mononuclear cell therapy in a porcine model of chronically infarcted myocardium. Cardiovasc Radiat Med. 2004;5:125–131. - PubMed
-
- Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. - PMC - PubMed
-
- Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. - PMC - PubMed
-
- Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R37 HL055757/HL/NHLBI NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- HL-91202/HL/NHLBI NIH HHS/United States
- R01 HL070897/HL/NHLBI NIH HHS/United States
- R01-HL-68088/HL/NHLBI NIH HHS/United States
- R01 HL091202/HL/NHLBI NIH HHS/United States
- HL-55757/HL/NHLBI NIH HHS/United States
- R01 HL074351/HL/NHLBI NIH HHS/United States
- R01 HL076794/HL/NHLBI NIH HHS/United States
- G0300395/MRC_/Medical Research Council/United Kingdom
- P01 AG043353/AG/NIA NIH HHS/United States
- HL-78825/HL/NHLBI NIH HHS/United States
- R01 HL055757/HL/NHLBI NIH HHS/United States
- HL-70897/HL/NHLBI NIH HHS/United States
- R01 HL114346/HL/NHLBI NIH HHS/United States
- HL-74351/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- P01 HL078825/HL/NHLBI NIH HHS/United States
- HL-76794/HL/NHLBI NIH HHS/United States
- R01 HL068088/HL/NHLBI NIH HHS/United States
- P20 GM103527/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

