An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced proneoplastic potential
- PMID: 23757312
- PMCID: PMC3753575
- DOI: 10.1161/CIRCULATIONAHA.113.002203
An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced proneoplastic potential
Abstract
Background: Doxorubicin (DOXO) is an effective anthracycline chemotherapeutic, but its use is limited by cumulative dose-dependent cardiotoxicity. Neuregulin-1β is an ErbB receptor family ligand that is effective against DOXO-induced cardiomyopathy in experimental models but is also proneoplastic. We previously showed that an engineered bivalent neuregulin-1β (NN) has reduced proneoplastic potential in comparison with the epidermal growth factor-like domain of neuregulin-1β (NRG), an effect mediated by receptor biasing toward ErbB3 homotypic interactions uncommonly formed by native neuregulin-1β. Here, we hypothesized that a newly formulated, covalent NN would be cardioprotective with reduced proneoplastic effects in comparison with NRG.
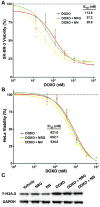
Methods and results: NN was expressed as a maltose-binding protein fusion in Escherichia coli. As established previously, NN stimulated antineoplastic or cytostatic signaling and phenotype in cancer cells, whereas NRG stimulated proneoplastic signaling and phenotype. In neonatal rat cardiomyocytes, NN and NRG induced similar downstream signaling. NN, like NRG, attenuated the double-stranded DNA breaks associated with DOXO exposure in neonatal rat cardiomyocytes and human cardiomyocytes derived from induced pluripotent stem cells. NN treatment significantly attenuated DOXO-induced decrease in fractional shortening as measured by blinded echocardiography in mice in a chronic cardiomyopathy model (57.7±0.6% versus 50.9±2.6%, P=0.004), whereas native NRG had no significant effect (49.4±3.7% versus 50.9±2.6%, P=0.813).
Conclusions: NN is a cardioprotective agent that promotes cardiomyocyte survival and improves cardiac function in DOXO-induced cardiotoxicity. Given the reduced proneoplastic potential of NN versus NRG, NN has translational potential for cardioprotection in patients with cancer receiving anthracyclines.
Keywords: anthracyclines; protein engineering; translational cancer chemotherapy protocols.
Conflict of interest statement
Figures
Comment in
-
Mechanism-based engineering against anthracycline cardiotoxicity.Circulation. 2013 Jul 9;128(2):98-100. doi: 10.1161/CIRCULATIONAHA.113.003688. Epub 2013 Jun 11. Circulation. 2013. PMID: 23757311 Free PMC article. No abstract available.
References
-
- Gennari A, Sormani MP, Pronzato P, Puntoni M, Colozza M, Pfeffer U, Bruzzi P. HER2 Status and Efficacy of Adjuvant Anthracyclines in Early Breast Cancer: A Pooled Analysis of Randomized Trials. J Natl Cancer Inst. 2008;100:14–20. - PubMed
-
- Wouters KA, Kremer LCM, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561–578. - PubMed
-
- Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. - PubMed
-
- Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. - PubMed
-
- Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG032977/AG/NIA NIH HHS/United States
- DK090147/DK/NIDDK NIH HHS/United States
- HL112905/HL/NHLBI NIH HHS/United States
- K99 HL112905/HL/NHLBI NIH HHS/United States
- U54 CA112967/CA/NCI NIH HHS/United States
- R01 EB003805/EB/NIBIB NIH HHS/United States
- R01 HL109264/HL/NHLBI NIH HHS/United States
- EB003805/EB/NIBIB NIH HHS/United States
- U54-CA112967/CA/NCI NIH HHS/United States
- R00 HL112905/HL/NHLBI NIH HHS/United States
- AG032977/AG/NIA NIH HHS/United States
- K08 DK090147/DK/NIDDK NIH HHS/United States
- DE019523/DE/NIDCR NIH HHS/United States
- R01 DE019523/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

