Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation
- PMID: 23757408
- PMCID: PMC3742854
- DOI: 10.1152/ajpendo.00006.2013
Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation
Abstract
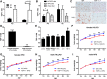
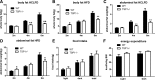
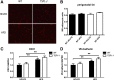
As a typical matricellular protein, thrombospondin (TSP)-1, binds to the structural matrix and regulates cellular behavior by modulating growth factor and cytokine signaling. Obesity and diabetes are associated with marked upregulation of TSP-1 in adipose tissue. We hypothesized that endogenous TSP-1 may play an important role in the pathogenesis of diet-induced obesity and metabolic dysfunction. Accordingly, we examined the effects of TSP-1 gene disruption on weight gain, adiposity, and adipose tissue inflammation in mice receiving a high-fat diet (HFD: 60% fat, 20% carbohydrate) or a high-carbohydrate low-fat diet (HCLFD: 10% fat, 70% carbohydrate). HFD mice had significantly higher TSP-1 expression in perigonadal adipose tissue; TSP-1 was predominantly localized in the adipose interstitium. TSP-1 loss attenuated weight gain and fat accumulation in HFD and HCLFD groups. Compared with corresponding wild-type animals, TSP-1-null mice had decreased insulin levels but exhibited elevated free fatty acid and triglyceride levels, suggesting impaired fatty acid uptake. TSP-1 loss did not affect adipocyte size and had no effect on adipose vascular density. However, TSP-1-null mice exhibited attenuated tumor necrosis factor-α mRNA expression and reduced macrophage infiltration, suggesting a role for TSP-1 in mediating obesity-associated inflammation. In vitro, TSP-1 enhanced proliferation of 3T3-L1 preadipocytes but did not modulate inflammatory cytokine and chemokine synthesis. In conclusion, TSP-1 upregulation contributes to weight gain, adipose growth, and the pathogenesis of metabolic dysfunction. The effects of TSP-1 may involve stimulation of adipocyte proliferation, activation of inflammatory signaling, and facilitated fatty acid uptake by adipocytes.
Keywords: adipocyte; inflammation; macrophage; matricellular proteins; obesity.
Figures

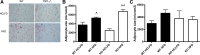


References
-
- Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Fruhbeck G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J Clin Endocrinol Metab 97: E1880–E1889, 2012 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

