Lineage-dependent circuit assembly in the neocortex
- PMID: 23757410
- PMCID: PMC3678337
- DOI: 10.1242/dev.087668
Lineage-dependent circuit assembly in the neocortex
Abstract
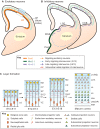
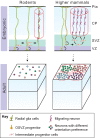
The neocortex plays a key role in higher-order brain functions, such as perception, language and decision-making. Since the groundbreaking work of Ramón y Cajal over a century ago, defining the neural circuits underlying brain functions has been a field of intense study. Here, we review recent findings on the formation of neocortical circuits, which have taken advantage of improvements to mouse genetics and circuit-mapping tools. These findings are beginning to reveal how individual components of circuits are generated and assembled during development, and how early developmental processes, such as neurogenesis and neuronal migration, guide precise circuit assembly.
Keywords: Lineage; Neocortex; Neuronal circuits.
Figures

References
-
- Abeles M., Goldstein M. H., Jr (1970). Functional architecture in cat primary auditory cortex: columnar organization and organization according to depth. J. Neurophysiol. 33, 172–187 - PubMed
-
- Anderson S. A., Eisenstat D. D., Shi L., Rubenstein J. L. (1997). Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476 - PubMed
-
- Anderson S. A., Kaznowski C. E., Horn C., Rubenstein J. L. R., McConnell S. K. (2002). Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb. Cortex 12, 702–709 - PubMed
-
- Angevine J. B., Jr, Sidman R. L. (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

