Molecular architecture of the uncleaved HIV-1 envelope glycoprotein trimer
- PMID: 23757493
- PMCID: PMC3725050
- DOI: 10.1073/pnas.1307382110
Molecular architecture of the uncleaved HIV-1 envelope glycoprotein trimer
Abstract
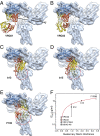
The human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) trimer, a membrane-fusing machine, mediates virus entry into host cells and is the sole virus-specific target for neutralizing antibodies. Binding the receptors, CD4 and CCR5/CXCR4, triggers Env conformational changes from the metastable unliganded state to the fusion-active state. We used cryo-electron microscopy to obtain a 6-Å structure of the membrane-bound, heavily glycosylated HIV-1 Env trimer in its uncleaved and unliganded state. The spatial organization of secondary structure elements reveals that the unliganded conformations of both glycoprotein (gp)120 and gp41 subunits differ from those induced by receptor binding. The gp120 trimer association domains, which contribute to interprotomer contacts in the unliganded Env trimer, undergo rearrangement upon CD4 binding. In the unliganded Env, intersubunit interactions maintain the gp41 ectodomain helical bundles in a "spring-loaded" conformation distinct from the extended helical coils of the fusion-active state. Quaternary structure regulates the virus-neutralizing potency of antibodies targeting the conserved CD4-binding site on gp120. The Env trimer architecture provides mechanistic insights into the metastability of the unliganded state, receptor-induced conformational changes, and quaternary structure-based strategies for immune evasion.
Keywords: cryo-EM; membrane protein; retrovirus; spike; vaccine immunogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
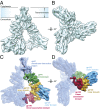
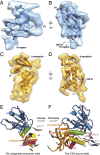
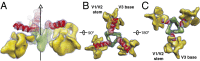

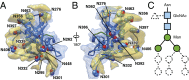
Comment in
-
Finding trimeric HIV-1 envelope glycoproteins in random noise.Proc Natl Acad Sci U S A. 2013 Nov 5;110(45):E4175-7. doi: 10.1073/pnas.1314353110. Epub 2013 Oct 8. Proc Natl Acad Sci U S A. 2013. PMID: 24106301 Free PMC article. No abstract available.
-
Structure of trimeric HIV-1 envelope glycoproteins.Proc Natl Acad Sci U S A. 2013 Nov 5;110(45):E4172-4. doi: 10.1073/pnas.1313802110. Epub 2013 Oct 8. Proc Natl Acad Sci U S A. 2013. PMID: 24106302 Free PMC article. No abstract available.
-
Avoiding the pitfalls of single particle cryo-electron microscopy: Einstein from noise.Proc Natl Acad Sci U S A. 2013 Nov 5;110(45):18037-41. doi: 10.1073/pnas.1314449110. Epub 2013 Oct 8. Proc Natl Acad Sci U S A. 2013. PMID: 24106306 Free PMC article.
-
Reply to Subramaniam, van Heel, and Henderson: Validity of the cryo-electron microscopy structures of the HIV-1 envelope glycoprotein complex.Proc Natl Acad Sci U S A. 2013 Nov 5;110(45):E4178-82. doi: 10.1073/pnas.1316666110. Proc Natl Acad Sci U S A. 2013. PMID: 24350339 Free PMC article. No abstract available.
References
-
- Barré-Sinoussi F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. - PubMed
-
- Gallo RC, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224(4648):500–503. - PubMed
-
- Allan JS, et al. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985;228(4703):1091–1094. - PubMed
-
- Robey WG, et al. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985;228(4699):593–595. - PubMed
-
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science. 1998;280(5371):1884–1888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

