Developing high-fidelity hepatotoxicity models from pluripotent stem cells
- PMID: 23757504
- PMCID: PMC3697818
- DOI: 10.5966/sctm.2012-0138
Developing high-fidelity hepatotoxicity models from pluripotent stem cells
Abstract
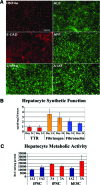
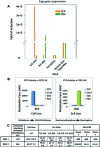
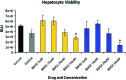
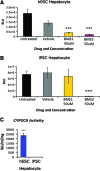
Faithfully recapitulating human physiology "in a dish" from a renewable source remains a holy grail for medicine and pharma. Many procedures have been described that, to a limited extent, exhibit human tissue-specific function in vitro. In particular, incomplete cellular differentiation and/or the loss of cell phenotype postdifferentiation play a major part in this void. We have developed an interdisciplinary approach to address this problem, using skill sets in cell biology, materials chemistry, and pharmacology. Pluripotent stem cells were differentiated to hepatocytes before being replated onto a synthetic surface. Our approach yielded metabolically active hepatocyte populations that displayed stable function for more than 2 weeks in vitro. Although metabolic activity was an important indication of cell utility, the accurate prediction of cellular toxicity in response to specific pharmacological compounds represented our goal. Therefore, detailed analysis of hepatocellular toxicity was performed in response to a custom-built and well-defined compound set and compared with primary human hepatocytes. Importantly, stem cell-derived hepatocytes displayed equivalence to primary human material. Moreover, we demonstrated that our approach was capable of modeling metabolic differences observed in the population. In conclusion, we report that pluripotent stem cell-derived hepatocytes will model toxicity predictably and in a manner comparable to current gold standard assays, representing a major advance in the field.
Keywords: Embryonic stem cells; Hepatocyte differentiation; Stem cell; Toxicity; iPS.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

