Cytotoxicity of 1,4-diamino-2-butanone, a putrescine analogue, to RKO cells: mechanism and redox imbalance
- PMID: 23758064
- PMCID: PMC6525713
- DOI: 10.3109/10715762.2013.814126
Cytotoxicity of 1,4-diamino-2-butanone, a putrescine analogue, to RKO cells: mechanism and redox imbalance
Abstract
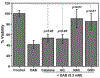
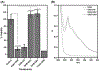
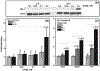
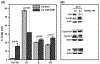
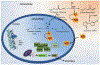
α-Aminocarbonyl metabolites (e.g., 5-aminolevulinic acid and aminoacetone) and the wide spectrum microbicide 1,4-diamino-2-butanone (DAB) have been shown to exhibit pro-oxidant properties. In vitro, these compounds undergo phosphate-catalyzed enolization at physiological pH and subsequent superoxide radical-propagated aerobic oxidation, yielding a reactive α-oxoaldehyde and H2O2. DAB cytotoxicity to pathogenic microorganisms has been attributed to the inhibition of polyamine biosynthesis. However, the role played in cell death by reactive DAB oxidation products is still poorly understood. This work aims to clarify the mechanism of DAB-promoted pro-oxidant action on mammalian cells. DAB (0.05-10 mM) treatment of RKO cells derived from human colon carcinoma led to a decrease in cell viability (IC50 ca. 0.3 mM DAB, 24 h incubation). Pre-addition of either catalase (5 μM) or aminoguanidine (20 mM) was observed to partially inhibit the toxic effects of DAB to the cells, while N-acetyl-L-cysteine (NAC, 5 mM) or reduced glutathione (GSH, 5 mM) provided almost complete protection against DAB. Changes in redox balance and stress response pathways were indicated by the increased expression of HO-1, NQO1 and xCT. Moreover, the observation of caspase 3 and PARP cleavage products is consistent with DAB-triggered apoptosis in RKO cells, which was corroborated by the partial protection afforded by the pan-caspase inhibitor z-VAD-FMK. Finally, DAB treatment disrupted the cell cycle in response to increased p53 and activation of ATM. Altogether, these data support the hypothesis that DAB exerts cytotoxicity via a mechanism involving not only polyamine biosynthesis but also by DAB oxidation products.
Conflict of interest statement
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Figures

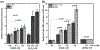
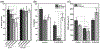

Similar articles
-
1,4-Diamino-2-butanone, a wide-spectrum microbicide, yields reactive species by metal-catalyzed oxidation.Free Radic Biol Med. 2011 Jun 15;50(12):1760-70. doi: 10.1016/j.freeradbiomed.2011.03.033. Epub 2011 Apr 3. Free Radic Biol Med. 2011. PMID: 21466850
-
1,4-Diamino-2-butanone, a putrescine analogue, promotes redox imbalance in Trypanosoma cruzi and mammalian cells.Arch Biochem Biophys. 2012 Dec 15;528(2):103-10. doi: 10.1016/j.abb.2012.09.005. Epub 2012 Oct 2. Arch Biochem Biophys. 2012. PMID: 23036870
-
The putrescine analogue 1,4-diamino-2-butanone affects polyamine synthesis, transport, ultrastructure and intracellular survival in Leishmania amazonensis.Microbiology (Reading). 2008 Oct;154(Pt 10):3104-3111. doi: 10.1099/mic.0.2007/013896-0. Microbiology (Reading). 2008. PMID: 18832316
-
Effects of a putrescine analog on Giardia lamblia.Parasitol Res. 2008 Jul;103(2):363-70. doi: 10.1007/s00436-008-0981-9. Epub 2008 Apr 24. Parasitol Res. 2008. PMID: 18437421
-
The variable chemotherapeutic response of Malabaricone-A in leukemic and solid tumor cell lines depends on the degree of redox imbalance.Phytomedicine. 2015 Jul 15;22(7-8):713-23. doi: 10.1016/j.phymed.2015.05.007. Epub 2015 May 29. Phytomedicine. 2015. PMID: 26141757
Cited by
-
Interferon gamma-induced apoptosis of head and neck squamous cell carcinoma is connected to indoleamine-2,3-dioxygenase via mitochondrial and ER stress-associated pathways.Cell Div. 2016 Aug 2;11:11. doi: 10.1186/s13008-016-0023-4. eCollection 2016. Cell Div. 2016. PMID: 27486476 Free PMC article.
References
-
- Menezes D, Valentim C, Oliveira MF, Vannier-santos MA. Putrescine analogue cytotoxicity against Trypanosoma cruzi. Parasitol Res 2006; 98: 99–105. - PubMed
-
- Soares CO, Colli W, Alves MJM, Bechara EJH. 1,4-Diamino-2-butanone a putrescine analogue promotes redox imbalance in Trypanosoma cruzi and mammalian cells. Arch Biochem Biophys 2012; 528: 103–107. - PubMed
-
- Ueno Y, Fukumatsu M, Ogasawara A, Watanabe T, Mikami T, Matsumoto T. Hyphae formation of Candida albicans is regulated by polyamines. Biol Pharm Bull 2004; 27: 890–892. - PubMed
-
- Maia C, Lanfredi-Rangel A, Santana-Anjos KG, Oliveira MF, Souza W, Vannier-Santos MA. Effects of a putrescine analog on Giardia lamblia. Parasitol Res 2008; 103: 363–37. - PubMed
-
- Pegg AE. Polyamine metabolism and its importance in neo-plastic growth and target for chemotherapy. Cancer Res 1988; 48: 759–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
