A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia
- PMID: 23758736
- PMCID: PMC3688296
- DOI: 10.1186/1479-5876-11-143
A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia
Abstract
Background: Peripheral vascular disease of the lower extremities comprises a clinical spectrum that extends from no symptoms to presentation with critical limb ischemia (CLI). Bone marrow derived Mesenchymal Stem Cells (BM- MSCs) may ameliorate the consequences of CLI due to their combinatorial potential for inducing angiogenesis and immunomodulatory environment in situ. The primary objective was to determine the safety of BM- MSCs in patients with CLI.
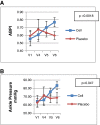
Methods: Prospective, double blind randomized placebo controlled multi-center study was conducted in patients with established CLI as per Rutherford classification in category II-4, III-5, or III-6 with infra-inguinal arterial occlusive disease and were not suitable for or had failed revascularization treatment. The primary end point was incidence of treatment - related adverse events (AE). Exploratory efficacy end points were improvement in rest pain, increase in Ankle Brachial Pressure Index (ABPI), ankle pressure, healing of ulcers, and amputation rates. Twenty patients (BM-MSC: Placebo = 1:1) were administered with allogeneic BM-MSCs at a dose of 2 million cells/kg or placebo (PlasmaLyte A) at the gastrocnemius muscle of the ischemic limb.
Results: Improvement was observed in the rest pain scores in both the arms. Significant increase in ABPI and ankle pressure was seen in BM-MSC arm compared to the placebo group. Incidence of AEs in the BM-MSC arm was 13 vs. 45 in the placebo arm where as serious adverse events (SAE) were similar in both the arms (5 in BM-MSC and 4 in the placebo group). SAEs resulted in death, infected gangrene, amputations in these patients. It was observed that the SAEs were related to disease progression and not related to stem cells.
Conclusion: BM-MSCs are safe when injected IM at a dose of 2 million cells/kg body weight. Few efficacy parameters such as ABPI and ankle pressure showed positive trend warranting further studies.
Trial registration: NIH website (http://www.clinicaltrials.gov/ct2/show/NCT00883870).
Figures


Comment in
-
Mesenchymal stromal cells for the treatment of critical limb ischemia: context and perspective.Stem Cell Res Ther. 2013;4(6):140. doi: 10.1186/scrt351. Stem Cell Res Ther. 2013. PMID: 24246031 Free PMC article.
References
-
- Sprengers RW, Lips DJ, Moll FL, Verhaar MC. Progenitor cell therapy in patients with critical limb ischemia without surgical options. Ann Surg. 2008;13:411–420. - PubMed
-
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease) endorsed by the American association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; TransAtlantic inter-society consensus; and vascular disease foundation. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. - DOI - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. - PubMed
-
- The European Agency for the Evaluation of Medicinal Products. Committee for Proprietary Medicinal Products. Note for guidance on clinical investigation of medicinal products for the treatment of peripheral arterial occlusive disease. CPMP/EWP/714/98 REV. 2002;1:1–16.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

