Down-regulation of the cancer/testis antigen 45 (CT45) is associated with altered tumor cell morphology, adhesion and migration
- PMID: 23758873
- PMCID: PMC3689639
- DOI: 10.1186/1478-811X-11-41
Down-regulation of the cancer/testis antigen 45 (CT45) is associated with altered tumor cell morphology, adhesion and migration
Abstract
Background: Due to their restricted expression in male germ cells and certain tumors, cancer/testis (CT) antigens are regarded as promising targets for tumor therapy. CT45 is a recently identified nuclear CT antigen that was associated with a severe disease score in Hodgkin's lymphoma and poor prognosis in multiple myeloma. As for many CT antigens, the biological function of CT45 in developing germ cells and in tumor cells is largely unknown.
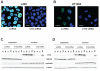
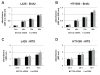
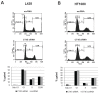
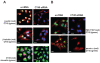
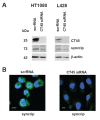
Methods: CT45 expression was down-regulated in CT45-positive Hodgkin's lymphoma (L428), fibrosarcoma (HT1080) and myeloma (U266B1) cells using RNA interference. An efficient CT45 knock-down was confirmed by immunofluorescence staining and/or Western blotting. These cellular systems allowed us to analyze the impact of CT45 down-regulation on proliferation, cell cycle progression, morphology, adhesion, migration and invasive capacity of tumor cells.
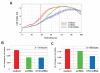
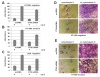
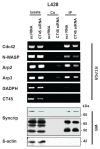
Results: Reduced levels of CT45 did not coincide with changes in cell cycle progression or proliferation. However, we observed alterations in cell adherence, morphology and migration/invasion after CT45 down-regulation. Significant changes in the distribution of cytoskeleton-associated proteins were detected by confocal imaging. Changes in cell adherence were recorded in real-time using the xCelligence system with control and siRNA-treated cells. Altered migratory and invasive capacity of CT45 siRNA-treated cells were visualized in 3D migration and invasion assays. Moreover, we found that CT45 down-regulation altered the level of the heterogeneous nuclear ribonucleoprotein syncrip (hnRNP-Q1) which is known to be involved in the control of focal adhesion formation and cell motility.
Conclusions: Providing first evidence of a cell biological function of CT45, we suggest that this cancer/testis antigen is involved in the modulation of cell morphology, cell adherence and cell motility. Enhanced motility and/or invasiveness of CT45-positive cells could contribute to the more severe disease progression that is correlated to CT45-positivity in several malignancies.
Figures
Similar articles
-
DNA hypomethylation-mediated activation of Cancer/Testis Antigen 45 (CT45) genes is associated with disease progression and reduced survival in epithelial ovarian cancer.Epigenetics. 2015;10(8):736-48. doi: 10.1080/15592294.2015.1062206. Epigenetics. 2015. PMID: 26098711 Free PMC article.
-
Characterization and expression of CT45 in Hodgkin's lymphoma.Clin Cancer Res. 2006 Aug 15;12(16):4804-11. doi: 10.1158/1078-0432.CCR-06-0186. Clin Cancer Res. 2006. PMID: 16914565
-
Cancer/testis antigen CT45: analysis of mRNA and protein expression in human cancer.Int J Cancer. 2009 Jun 15;124(12):2893-8. doi: 10.1002/ijc.24296. Int J Cancer. 2009. PMID: 19296537
-
Frequency and prognostic relevance of cancer testis antigen 45 expression in multiple myeloma.Exp Hematol. 2009 Apr;37(4):446-9. doi: 10.1016/j.exphem.2008.12.003. Epub 2009 Feb 10. Exp Hematol. 2009. PMID: 19211183
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
Cited by
-
DNA hypomethylation-mediated activation of Cancer/Testis Antigen 45 (CT45) genes is associated with disease progression and reduced survival in epithelial ovarian cancer.Epigenetics. 2015;10(8):736-48. doi: 10.1080/15592294.2015.1062206. Epigenetics. 2015. PMID: 26098711 Free PMC article.
-
Uev1A promotes breast cancer cell migration by up-regulating CT45A expression via the AKT pathway.BMC Cancer. 2021 Sep 9;21(1):1012. doi: 10.1186/s12885-021-08750-3. BMC Cancer. 2021. PMID: 34503444 Free PMC article.
-
The expression of cancer-testis antigen in ovarian cancer and the development of immunotherapy.Am J Cancer Res. 2022 Feb 15;12(2):681-694. eCollection 2022. Am J Cancer Res. 2022. PMID: 35261795 Free PMC article. Review.
-
Effects of size and surface of zinc oxide and aluminum-doped zinc oxide nanoparticles on cell viability inferred by proteomic analyses.Int J Nanomedicine. 2014 Aug 2;9:3631-43. doi: 10.2147/IJN.S66651. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25120361 Free PMC article.
-
Combined cancer testis antigens enhanced prediction accuracy for prognosis of patients with hepatocellular carcinoma.Int J Clin Exp Pathol. 2015 Apr 1;8(4):3513-28. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26097535 Free PMC article.
References
-
- Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. - PubMed
-
- Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A, Lehvaslaiho M, Carninci P, Hayashizaki Y, JongeneeL CV, Simpson AJG, Old LJ, Hide W. Genome-wide analysis of cancer/testis gene expression. PNAS. 2008;105:20422–27. doi: 10.1073/pnas.0810777105. - DOI - PMC - PubMed
-
- Almeida LG, Sakabe NJ, DeOliveira AR, Silva MCC, Mundstein AS, Cohen T, Chen YT, Chua R, Gurung S, Gnjatic S, Jungbluth AA, Caballero OL, Bairoch A, Kiesler E, White SL, Simpson AJG, Old LJ, Camargo AA, Vasconcelos AT. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37:D816–D819. doi: 10.1093/nar/gkn673. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

