Risk of bias of randomized controlled trials published in orthopaedic journals
- PMID: 23758875
- PMCID: PMC3724580
- DOI: 10.1186/1471-2288-13-76
Risk of bias of randomized controlled trials published in orthopaedic journals
Abstract
Background: The purpose of this study was to assess the quality of methodology in orthopaedics-related randomized controlled trials (RCTs) published from January 2006 to December 2010 in the top orthopaedic journals based on impact scores from the Thompson ISI citation reports (2010).
Methods: Journals included American Journal of Sports Medicine; Journal of Orthopaedic Research; Journal of Bone and Joint Surgery, American; Spine Journal; and Osteoarthritis and Cartilage. Each RCT was assessed on ten criteria (randomization method, allocation sequence concealment, participant blinding, outcome assessor blinding, outcome measurement, interventionist training, withdrawals, intent to treat analyses, clustering, and baseline characteristics) as having empirical evidence for biasing treatment effect estimates when not performed properly.
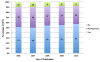
Results: A total of 232 RCTs met our inclusion criteria. The proportion of RCTs in published journals fell from 6% in 2006 to 4% in 2010. Forty-nine percent of the criteria were fulfilled across these journals, with 42% of the criteria not being amendable to assessment due to inadequate reporting. The results of our regression revealed that a more recent publication year was significantly associated with more fulfilled criteria (β = 0.171; CI = -0.00 to 0.342; p = 0.051).
Conclusion: In summary, very few studies met all ten criteria. Thus, many of these studies likely have biased estimates of treatment effects. In addition, these journals had poor reporting of important methodological aspects.
Figures
References
-
- Mulrow CD, Cook DJ, Davidoff F. In: Systematic reviews: synthesis of best evidence for health-care decisions. Mulrow C, Cook D, editor. Philadelphia, PA: American College of Physicians; 1998. Systematic reviews: critical links in the great chain of evidence.
-
- Sackett DL, Richardsom WS, Rosenberg W, Haynes B. Evidence-based medicine: how to practice and teach EBM. New York, NY: Churchill Livingstone; 1998.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous