Archaeal nucleosome positioning in vivo and in vitro is directed by primary sequence motifs
- PMID: 23758892
- PMCID: PMC3691661
- DOI: 10.1186/1471-2164-14-391
Archaeal nucleosome positioning in vivo and in vitro is directed by primary sequence motifs
Abstract
Background: Histone wrapping of DNA into nucleosomes almost certainly evolved in the Archaea, and predates Eukaryotes. In Eukaryotes, nucleosome positioning plays a central role in regulating gene expression and is directed by primary sequence motifs that together form a nucleosome positioning code. The experiments reported were undertaken to determine if archaeal histone assembly conforms to the nucleosome positioning code.
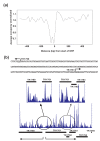
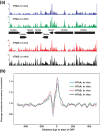
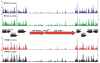
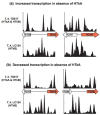
Results: Eukaryotic nucleosome positioning is favored and directed by phased helical repeats of AA/TT/AT/TA and CC/GG/CG/GC dinucleotides, and disfavored by longer AT-rich oligonucleotides. Deep sequencing of genomic DNA protected from micrococcal nuclease digestion by assembly into archaeal nucleosomes has established that archaeal nucleosome assembly is also directed and positioned by these sequence motifs, both in vivo in Methanothermobacter thermautotrophicus and Thermococcus kodakarensis and in vitro in reaction mixtures containing only one purified archaeal histone and genomic DNA. Archaeal nucleosomes assembled at the same locations in vivo and in vitro, with much reduced assembly immediately upstream of open reading frames and throughout the ribosomal rDNA operons. Providing further support for a common positioning code, archaeal histones assembled into nucleosomes on eukaryotic DNA and eukaryotic histones into nucleosomes on archaeal DNA at the same locations. T. kodakarensis has two histones, designated HTkA and HTkB, and strains with either but not both histones deleted grow normally but do exhibit transcriptome differences. Comparisons of the archaeal nucleosome profiles in the intergenic regions immediately upstream of genes that exhibited increased or decreased transcription in the absence of HTkA or HTkB revealed substantial differences but no consistent pattern of changes that would correlate directly with archaeal nucleosome positioning inhibiting or stimulating transcription.
Conclusions: The results obtained establish that an archaeal histone and a genome sequence together are sufficient to determine where archaeal nucleosomes preferentially assemble and where they avoid assembly. We confirm that the same nucleosome positioning code operates in Archaea as in Eukaryotes and presumably therefore evolved with the histone-fold mechanism of DNA binding and compaction early in the archaeal lineage, before the divergence of Eukaryotes.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

