A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies
- PMID: 23758961
- PMCID: PMC3691846
- DOI: 10.1186/1745-6215-14-166
A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies
Abstract
Background: Randomised controlled trials (RCTs) are the gold standard assessment for health technologies. A key aspect of the design of any clinical trial is the target sample size. However, many publicly-funded trials fail to reach their target sample size. This study seeks to assess the current state of recruitment success and grant extensions in trials funded by the Health Technology Assessment (HTA) program and the UK Medical Research Council (MRC).
Methods: Data were gathered from two sources: the National Institute for Health Research (NIHR) HTA Journal Archive and the MRC subset of the International Standard Randomised Controlled Trial Number (ISRCTN) register. A total of 440 trials recruiting between 2002 and 2008 were assessed for eligibility, of which 73 met the inclusion criteria. Where data were unavailable from the reports, members of the trial team were contacted to ensure completeness.
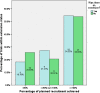
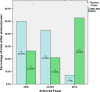
Results: Over half (55%) of trials recruited their originally specified target sample size, with over three-quarters (78%) recruiting 80% of their target. There was no evidence of this improving over the time of the assessment. Nearly half (45%) of trials received an extension of some kind. Those that did were no more likely to successfully recruit. Trials with 80% power were less likely to successfully recruit compared to studies with 90% power.
Conclusions: While recruitment appears to have improved since 1994 to 2002, publicly-funded trials in the UK still struggle to recruit to their target sample size, and both time and financial extensions are often requested. Strategies to cope with such problems should be more widely applied. It is recommended that where possible studies are planned with 90% power.
Figures
References
-
- Julious SA. Sample Sizes for Clinical Trials. Boca Raton, FL: CRC Press; 2010.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous