Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers
- PMID: 23758976
- PMCID: PMC3698035
- DOI: 10.1186/1476-4598-12-60
Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers
Abstract
Background: Nectin-2 is a Ca(2+)-independent cell-cell adhesion molecule that is one of the plasma membrane components of adherens junctions. However, little has been reported about the involvement of Nectin-2 in cancer.
Methods: To determine the expression of Nectin-2 in cancer tissues and cancer cell lines, we performed gene expression profile analysis, immunohistochemistry studies, and flow cytometry analysis. We also investigated the potential of this molecule as a target for antibody therapeutics to treat cancers by generating and characterizing an anti-Nectin-2 rabbit polyclonal antibody (poAb) and 256 fully human anti-Nectin-2 monoclonal antibodies (mAbs). In addition, we tested anti-Nectin-2 mAbs in several in vivo tumor growth inhibition models to investigate the primary mechanisms of action of the mAbs.
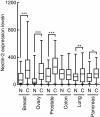
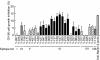
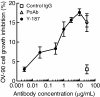
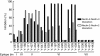
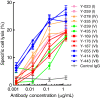
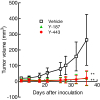
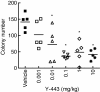
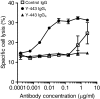
Results: In the present study, we found that Nectin-2 was over-expressed in clinical breast and ovarian cancer tissues by using gene expression profile analysis and immunohistochemistry studies. Nectin-2 was over-expressed in various cancer cell lines as well. Furthermore, the polyclonal antibody specific to Nectin-2 suppressed the in vitro proliferation of OV-90 ovarian cancer cells, which express endogenous Nectin-2 on the cell surface. The anti-Nectin-2 mAbs we generated were classified into 7 epitope bins. The anti-Nectin-2 mAbs demonstrated antibody-dependent cellular cytotoxicity (ADCC) and epitope bin-dependent features such as the inhibition of Nectin-2-Nectin-2 interaction, Nectin-2-Nectin-3 interaction, and in vitro cancer cell proliferation. A representative anti-Nectin-2 mAb in epitope bin VII, Y-443, showed anti-tumor effects against OV-90 cells and MDA-MB-231 breast cancer cells in mouse therapeutic models, and its main mechanism of action appeared to be ADCC.
Conclusions: We observed the over-expression of Nectin-2 in breast and ovarian cancers and anti-tumor activity of anti-Nectin-2 mAbs via strong ADCC. These findings suggest that Nectin-2 is a potential target for antibody therapy against breast and ovarian cancers.
Figures

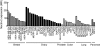



References
-
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

