Conversion of fatty aldehydes into alk (a/e)nes by in vitro reconstituted cyanobacterial aldehyde-deformylating oxygenase with the cognate electron transfer system
- PMID: 23759169
- PMCID: PMC3691600
- DOI: 10.1186/1754-6834-6-86
Conversion of fatty aldehydes into alk (a/e)nes by in vitro reconstituted cyanobacterial aldehyde-deformylating oxygenase with the cognate electron transfer system
Abstract
Background: Biosynthesis of fatty alk(a/e)ne in cyanobacteria has been considered as a potential basis for the sunlight-driven and carbon-neutral bioprocess producing advanced solar biofuels. Aldehyde-deformylating oxygenase (ADO) is a key enzyme involved in that pathway. The heterologous or chemical reducing systems were generally used in in vitro ADO activity assay. The cognate electron transfer system from cyanobacteria to support ADO activity is still unknown.
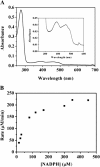
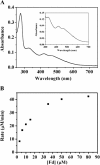
Results: We identified the potential endogenous reducing system including ferredoxin (Fd) and ferredoxin-NADP+ reductase (FNR) to support ADO activity in Synechococcus elongatus PCC7942. ADO (Synpcc7942_1593), FNR (SynPcc7942_0978), and Fd (SynPcc7942_1499) from PCC7942 were cloned, overexpressed, purified, and characterized. ADO activity was successfully supported with the endogenous electron transfer system, which worked more effectively than the heterologous and chemical ones. The results of the hybrid Fd/FNR reducing systems demonstrated that ADO was selective against Fd. And it was observed that the cognate reducing system produced less H2O2 than the heterologous one by 33% during ADO-catalyzed reactions. Importantly, kcat value of ADO 1593 using the homologous Fd/FNR electron transfer system is 3.7-fold higher than the chemical one.
Conclusions: The cognate electron transfer system from cyanobacteria to support ADO activity was identified and characterized. For the first time, ADO was functionally in vitro reconstituted with the endogenous reducing system from cyanobacteria, which supported greater activity than the surrogate and chemical ones, and produced less H2O2 than the heterologous one. The identified Fd/FNR electron transfer system will be potentially useful for improving ADO activity and further enhancing the biosynthetic efficiency of hydrocarbon biofuels in cyanobacteria.
Figures
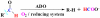


Similar articles
-
Structure-oriented substrate specificity engineering of aldehyde-deformylating oxygenase towards aldehydes carbon chain length.Biotechnol Biofuels. 2016 Aug 31;9(1):185. doi: 10.1186/s13068-016-0596-9. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27588038 Free PMC article.
-
Efficient delivery of long-chain fatty aldehydes from the Nostoc punctiforme acyl-acyl carrier protein reductase to its cognate aldehyde-deformylating oxygenase.Biochemistry. 2015 Feb 3;54(4):1006-15. doi: 10.1021/bi500847u. Epub 2015 Jan 22. Biochemistry. 2015. PMID: 25496470
-
Alka(e)ne synthesis in Cupriavidus necator boosted by the expression of endogenous and heterologous ferredoxin-ferredoxin reductase systems.Biotechnol Bioeng. 2018 Oct;115(10):2576-2584. doi: 10.1002/bit.26805. Epub 2018 Aug 29. Biotechnol Bioeng. 2018. PMID: 30063082
-
Recent advances in the improvement of cyanobacterial enzymes for bioalkane production.Microb Cell Fact. 2022 Dec 12;21(1):256. doi: 10.1186/s12934-022-01981-4. Microb Cell Fact. 2022. PMID: 36503511 Free PMC article. Review.
-
Cyanobacterial Enzymes for Bioalkane Production.Adv Exp Med Biol. 2018;1080:119-154. doi: 10.1007/978-981-13-0854-3_6. Adv Exp Med Biol. 2018. PMID: 30091094 Review.
Cited by
-
Structure-oriented substrate specificity engineering of aldehyde-deformylating oxygenase towards aldehydes carbon chain length.Biotechnol Biofuels. 2016 Aug 31;9(1):185. doi: 10.1186/s13068-016-0596-9. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27588038 Free PMC article.
-
A microbial platform for renewable propane synthesis based on a fermentative butanol pathway.Biotechnol Biofuels. 2015 Apr 10;8:61. doi: 10.1186/s13068-015-0231-1. eCollection 2015. Biotechnol Biofuels. 2015. PMID: 25866563 Free PMC article.
-
A consensus-guided approach yields a heat-stable alkane-producing enzyme and identifies residues promoting thermostability.J Biol Chem. 2018 Jun 15;293(24):9148-9161. doi: 10.1074/jbc.RA117.000639. Epub 2018 Apr 9. J Biol Chem. 2018. PMID: 29632075 Free PMC article.
-
Identification of residues important for the activity of aldehyde-deformylating oxygenase through investigation into the structure-activity relationship.BMC Biotechnol. 2017 Mar 16;17(1):31. doi: 10.1186/s12896-017-0351-8. BMC Biotechnol. 2017. PMID: 28302170 Free PMC article.
-
Ferritin-Like Proteins: A Conserved Core for a Myriad of Enzyme Complexes.Subcell Biochem. 2022;99:109-153. doi: 10.1007/978-3-031-00793-4_4. Subcell Biochem. 2022. PMID: 36151375
References
-
- Daroch M, Geng S, Wang G. Recent advances in liquid biofuel production from algal feedstocks. Appl Energy. 2013;102:1371–1381.
LinkOut - more resources
Full Text Sources
Other Literature Sources

