Three-dimensional culture sensitizes epithelial ovarian cancer cells to EZH2 methyltransferase inhibition
- PMID: 23759589
- PMCID: PMC3737313
- DOI: 10.4161/cc.25163
Three-dimensional culture sensitizes epithelial ovarian cancer cells to EZH2 methyltransferase inhibition
Abstract
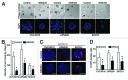
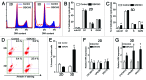
Inhibitors of EZH2 methyltransferase activity have been demonstrated to selectively suppress the growth of diffused large B cell lymphoma (DLBCL) cells with gain-of-function mutations in EZH2, while exhibiting very limited effects on the growth of DLBCL cells with wild-type EZH2. Given that EZH2 is often overexpressed but not mutated in solid tumors, it is important to investigate the determinants of sensitivity of solid tumor cells to EZH2 inhibitors. In the current study, we show that three-dimensional (3D) culture of epithelial ovarian cancer (EOC) cells that overexpress EZH2 sensitizes these cells to EZH2 methyltransferase inhibition. Treatment of EOC cells with GSK343, a specific inhibitor of EZH2 methyltransferase, decreases the level of H3K27Me3, the product of EZH2's enzymatic activity. However, GSK343 exhibited limited effects on the growth of EOC cells in conventional two-dimensional (2D) culture. In contrast, GSK343 significantly suppressed the growth of EOC cells cultured in 3D matrigel extracellular matrix (ECM), which more closely mimics the tumor microenvironment in vivo. Notably, GSK343 induces apoptosis of EOC cells in 3D but not 2D culture. In addition, GSK343 significantly inhibited the invasion of EOC cells. In summary, we show that the 3D ECM sensitizes EOC cells to EZH2 methyltransferase inhibition, which suppresses cell growth, induces apoptosis and inhibits invasion. Our findings imply that in EZH2 wild-type solid tumors, the ECM tumor microenvironment plays an important role in determining sensitivity to EZH2 inhibition and suggest that targeting the ECM represents a novel strategy for enhancing EZH2 inhibitor efficacy.
Keywords: 3D culture; EZH2; EZH2 inhibitor GSK343; apoptosis; epithelial ovarian cancer.
Figures


Comment in
-
3D culture adds an extra dimension to targeted epigenetic therapies.Cell Cycle. 2013 Jul 15;12(14):2173-4. doi: 10.4161/cc.25551. Cell Cycle. 2013. PMID: 23803725 Free PMC article. No abstract available.
-
Neighborhood matters: response to EZH2 methyltransferase inhibitors.Cell Cycle. 2013 Aug 1;12(15):2345. doi: 10.4161/cc.25775. Epub 2013 Jul 16. Cell Cycle. 2013. PMID: 23887388 Free PMC article. No abstract available.
References
-
- Atlanta: American Cancer Society Cancer Facts & Figures. 2012.
-
- Arulkumaran S, Regan L, Farquharson DIM. Obstetrics and gynaecology. Oxford: Oxford University Press, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
