Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells
- PMID: 23759711
- PMCID: PMC3979966
- DOI: 10.1016/j.scr.2013.05.002
Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells
Abstract
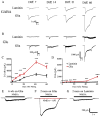
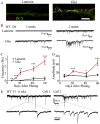
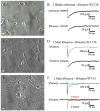
Neurons derived from human induced-pluripotent stem cells (hiPSCs) have been used to model a variety of neurological disorders. Different protocols have been used to differentiate hiPSCs into neurons, but their functional maturation process has varied greatly among different studies. Here, we demonstrate that laminin, a commonly used substrate for iPSC cultures, was inefficient to promote fully functional maturation of hiPSC-derived neurons. In contrast, astroglial substrate greatly accelerated neurodevelopmental processes of hiPSC-derived neurons. We have monitored the neural differentiation and maturation process for up to two months after plating hiPSC-derived neuroprogenitor cells (hNPCs) on laminin or astrocytes. We found that one week after plating hNPCs, there were 21-fold more newly differentiated neurons on astrocytes than on laminin. Two weeks after plating hNPCs, there were 12-fold more dendritic branches in neurons cultured on astrocytes than on laminin. Six weeks after plating hNPCs, the Na(+) and K(+) currents, as well as glutamate and GABA receptor currents, were 3-fold larger in neurons cultured on astrocytes than on laminin. And two months after plating hNPCs, the spontaneous synaptic events were 8-fold more in neurons cultured on astrocytes than on laminin. These results highlight a critical role of astrocytes in promoting neural differentiation and functional maturation of human neurons derived from hiPSCs. Moreover, our data presents a thorough developmental timeline of hiPSC-derived neurons in culture, providing important benchmarks for future studies on disease modeling and drug screening.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures







Similar articles
-
Role of Human-Induced Pluripotent Stem Cell-Derived Spinal Cord Astrocytes in the Functional Maturation of Motor Neurons in a Multielectrode Array System.Stem Cells Transl Med. 2019 Dec;8(12):1272-1285. doi: 10.1002/sctm.19-0147. Epub 2019 Oct 21. Stem Cells Transl Med. 2019. PMID: 31631575 Free PMC article.
-
Reprogramming of HUVECs into induced pluripotent stem cells (HiPSCs), generation and characterization of HiPSC-derived neurons and astrocytes.PLoS One. 2015 Mar 19;10(3):e0119617. doi: 10.1371/journal.pone.0119617. eCollection 2015. PLoS One. 2015. PMID: 25789622 Free PMC article.
-
Astrocyte-enriched feeder layers from cryopreserved cells support differentiation of spontaneously active networks of human iPSC-derived neurons.J Neurosci Methods. 2018 Jan 15;294:91-101. doi: 10.1016/j.jneumeth.2017.07.019. Epub 2017 Jul 23. J Neurosci Methods. 2018. PMID: 28746822 Free PMC article.
-
[Hypoxia epigenetically bestows astrocytic differentiation potential on human pluripotent cell-derived neural stem/precursor cells].Nihon Yakurigaku Zasshi. 2019;153(2):54-60. doi: 10.1254/fpj.153.54. Nihon Yakurigaku Zasshi. 2019. PMID: 30745514 Review. Japanese.
-
Modeling Neurological Disorders with Human Pluripotent Stem Cell-Derived Astrocytes.Int J Mol Sci. 2019 Aug 8;20(16):3862. doi: 10.3390/ijms20163862. Int J Mol Sci. 2019. PMID: 31398826 Free PMC article. Review.
Cited by
-
Electrical and chemical modulation of homogeneous and heterogeneous human-iPSCs-derived neuronal networks on high density arrays.Front Mol Neurosci. 2024 Feb 6;17:1304507. doi: 10.3389/fnmol.2024.1304507. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38380114 Free PMC article.
-
Chemical Conversion of Human Fetal Astrocytes into Neurons through Modulation of Multiple Signaling Pathways.Stem Cell Reports. 2019 Mar 5;12(3):488-501. doi: 10.1016/j.stemcr.2019.01.003. Epub 2019 Feb 7. Stem Cell Reports. 2019. PMID: 30745031 Free PMC article.
-
Combining NGN2 Programming with Developmental Patterning Generates Human Excitatory Neurons with NMDAR-Mediated Synaptic Transmission.Cell Rep. 2018 May 22;23(8):2509-2523. doi: 10.1016/j.celrep.2018.04.066. Cell Rep. 2018. PMID: 29791859 Free PMC article.
-
Novel Approaches Used to Examine and Control Neurogenesis in Parkinson's Disease.Int J Mol Sci. 2021 Sep 4;22(17):9608. doi: 10.3390/ijms22179608. Int J Mol Sci. 2021. PMID: 34502516 Free PMC article. Review.
-
Deriving Schwann cells from hPSCs enables disease modeling and drug discovery for diabetic peripheral neuropathy.Cell Stem Cell. 2023 May 4;30(5):632-647.e10. doi: 10.1016/j.stem.2023.04.006. Cell Stem Cell. 2023. PMID: 37146583 Free PMC article.
References
-
- Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. - PubMed
-
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. - PubMed
-
- Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, Park IH, Friedman BA, Daley GQ, Wyllie DJ, Hardingham GE, Wilmut I, Finkbeiner S, Maniatis T, Shaw CE, Chandran S. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci U S A. 2012;109(15):5803–5808. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- DP2 OD006495/OD/NIH HHS/United States
- 1R21MH093954-01A1/MH/NIMH NIH HHS/United States
- R01 MH094753/MH/NIMH NIH HHS/United States
- R01 MH083911/MH/NIMH NIH HHS/United States
- R01MH095741/MH/NIMH NIH HHS/United States
- R21 MH093954/MH/NIMH NIH HHS/United States
- P01 NICHD033113/PHS HHS/United States
- MH083911/MH/NIMH NIH HHS/United States
- R01 NH094753-02/NH/NIH HHS/United States
- R01 MH095741/MH/NIMH NIH HHS/United States
- 1-DP2-OD006495-01/OD/NIH HHS/United States
- MH092740/MH/NIMH NIH HHS/United States
- R21 MH092740/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

