Genetic engineering of yellow betalain pigments beyond the species barrier
- PMID: 23760173
- PMCID: PMC3679504
- DOI: 10.1038/srep01970
Genetic engineering of yellow betalain pigments beyond the species barrier
Abstract
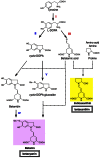
Betalains are one of the major plant pigment groups found in some higher plants and higher fungi. They are not produced naturally in any plant species outside of the order Caryophyllales, nor are they produced by anthocyanin-accumulating Caryophyllales. Here, we attempted to reconstruct the betalain biosynthetic pathway as a self-contained system in an anthocyanin-producing plant species. The combined expressions of a tyrosinase gene from shiitake mushroom and a DOPA 4,5-dioxygenase gene from the four-o'clock plant resulted in successful betalain production in cultured cells of tobacco BY2 and Arabidopsis T87. Transgenic tobacco BY2 cells were bright yellow because of the accumulation of betaxanthins. LC-TOF-MS analyses showed that proline-betaxanthin (Pro-Bx) accumulated as the major betaxanthin in these transgenic BY2 cells. Transgenic Arabidopsis T87 cells also produced betaxanthins, but produced lower levels than transgenic BY2 cells. These results illustrate the success of a novel genetic engineering strategy for betalain biosynthesis.
Figures



Similar articles
-
Betalain production is possible in anthocyanin-producing plant species given the presence of DOPA-dioxygenase and L-DOPA.BMC Plant Biol. 2012 Mar 12;12:34. doi: 10.1186/1471-2229-12-34. BMC Plant Biol. 2012. PMID: 22409631 Free PMC article.
-
Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants.New Phytol. 2016 Apr;210(1):269-83. doi: 10.1111/nph.13796. Epub 2015 Dec 18. New Phytol. 2016. PMID: 26683006
-
Flower color modification in Torenia fournieri by genetic engineering of betacyanin pigments.BMC Plant Biol. 2024 Jun 27;24(1):614. doi: 10.1186/s12870-024-05284-1. BMC Plant Biol. 2024. PMID: 38937670 Free PMC article.
-
The evolution of betalain biosynthesis in Caryophyllales.New Phytol. 2019 Oct;224(1):71-85. doi: 10.1111/nph.15980. Epub 2019 Jul 19. New Phytol. 2019. PMID: 31172524 Review.
-
Plant betalains: Chemistry and biochemistry.Phytochemistry. 2015 Sep;117:267-295. doi: 10.1016/j.phytochem.2015.06.008. Epub 2015 Jun 20. Phytochemistry. 2015. PMID: 26101148 Review.
Cited by
-
Additional betalain accumulation by genetic engineering leads to a novel flower color in lisianthus (Eustoma grandiflorum).Plant Biotechnol (Tokyo). 2021 Sep 25;38(3):323-330. doi: 10.5511/plantbiotechnology.21.0516a. Plant Biotechnol (Tokyo). 2021. PMID: 34782819 Free PMC article.
-
Proteomic Analysis of Hylocereus polyrhizus Reveals Metabolic Pathway Changes.Int J Mol Sci. 2016 Sep 28;17(10):1606. doi: 10.3390/ijms17101606. Int J Mol Sci. 2016. PMID: 27690004 Free PMC article.
-
Subcellular Localization of a Plant Catalase-Phenol Oxidase, AcCATPO, from Amaranthus and Identification of a Non-canonical Peroxisome Targeting Signal.Front Plant Sci. 2017 Aug 2;8:1345. doi: 10.3389/fpls.2017.01345. eCollection 2017. Front Plant Sci. 2017. PMID: 28824680 Free PMC article.
-
Tyrosine Hydroxylation in Betalain Pigment Biosynthesis Is Performed by Cytochrome P450 Enzymes in Beets (Beta vulgaris).PLoS One. 2016 Feb 18;11(2):e0149417. doi: 10.1371/journal.pone.0149417. eCollection 2016. PLoS One. 2016. PMID: 26890886 Free PMC article.
-
Application of natural photosensitizers in dye-sensitized solar cells: opportunities, challenges, and future outlook.Photochem Photobiol Sci. 2025 Aug;24(8):1443-1488. doi: 10.1007/s43630-025-00762-3. Epub 2025 Jul 20. Photochem Photobiol Sci. 2025. PMID: 40684421 Review.
References
-
- Grotewold E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 57, 761–780 (2006). - PubMed
-
- Tanaka Y., Sasaki N. & Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 54, 733–749 (2008). - PubMed
-
- Stafford H. A. Anthocyanins and betalains: evolution of the mutually exclusive pathways. Plant Sci. 101, 91–98 (1994).
-
- Hinz U. G., Fivaz J., Girod P. A. & Zyrd J. P. The gene coding for the DOPA dioxygenase involved in betalain biosynthesis in Amanita muscaria and its regulation. Mol. Gen. Genet. 256, 1–6 (1997). - PubMed
-
- Clement J. S. & Mabry T. J. Pigment evolution in the Caryophyllales: a systematic overview. Bot. Acta 109, 360–367 (1996).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

