Reinvestigating the role of IgM in rabies virus postexposure vaccination
- PMID: 23760250
- PMCID: PMC3754079
- DOI: 10.1128/JVI.00995-13
Reinvestigating the role of IgM in rabies virus postexposure vaccination
Abstract
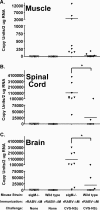
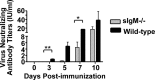
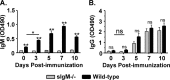
B cells secreting IgG antibodies, but not IgM, are thought to be solely responsible for vaccine-induced protection against rabies virus (RABV) infections in postexposure settings. In this report, we reinvestigated the potential for IgM to mediate protection in a mouse model of RABV vaccination. Immunocompetent mice immunized with an experimental live replication-deficient RABV-based vaccine produced virus neutralizing antibodies (VNAs) within 3 days of vaccination. However, mice unable to produce soluble IgM (sIgM(-/-)) did not produce VNAs until 7 days postimmunization. Furthermore, sIgM(-/-) mice were not protected against RABV infection when challenged 3 days postimmunization, while all wild-type mice survived challenge. Consistent with the lack of protection against pathogenic RABV challenge, approximately 50- to 100-fold higher viral loads of challenge virus were detected in the muscle, spinal cord, and brain of immunized sIgM(-/-) mice compared to control mice. In addition, IgG antibody titers in vaccinated wild-type and sIgM(-/-) mice were similar at all time points postimmunization, suggesting that protection against RABV challenge is due to the direct effects of IgM and not the influence of IgM on the development of effective IgG antibody titers. In all, early vaccine-induced IgM can limit dissemination of pathogenic RABV to the central nervous system and mediate protection against pathogenic RABV challenge. Considering the importance for the rapid induction of VNAs to protect against RABV infections in postexposure prophylaxis settings, these findings may help guide the development of a single-dose human rabies vaccine.
Figures

Similar articles
-
Targeting Vaccine-Induced Extrafollicular Pathway of B Cell Differentiation Improves Rabies Postexposure Prophylaxis.J Virol. 2017 Mar 29;91(8):e02435-16. doi: 10.1128/JVI.02435-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148792 Free PMC article.
-
Investigating the role for IL-21 in rabies virus vaccine-induced immunity.PLoS Negl Trop Dis. 2013;7(3):e2129. doi: 10.1371/journal.pntd.0002129. Epub 2013 Mar 14. PLoS Negl Trop Dis. 2013. PMID: 23516660 Free PMC article.
-
Virus-Like Vesicles Based on Semliki Forest Virus-Containing Rabies Virus Glycoprotein Make a Safe and Efficacious Rabies Vaccine Candidate in a Mouse Model.J Virol. 2021 Sep 27;95(20):e0079021. doi: 10.1128/JVI.00790-21. Epub 2021 Aug 4. J Virol. 2021. PMID: 34346765 Free PMC article.
-
The Immune Escape Strategy of Rabies Virus and Its Pathogenicity Mechanisms.Viruses. 2024 Nov 14;16(11):1774. doi: 10.3390/v16111774. Viruses. 2024. PMID: 39599888 Free PMC article. Review.
-
Lyssaviruses: current trends.Adv Virus Res. 2008;71:207-50. doi: 10.1016/S0065-3527(08)00005-5. Adv Virus Res. 2008. PMID: 18585530 Free PMC article. Review.
Cited by
-
Immunogenicity of replication-deficient vesicular stomatitis virus based rabies vaccine in mice.Vet Q. 2021 Dec;41(1):202-209. doi: 10.1080/01652176.2021.1930277. Vet Q. 2021. PMID: 33985418 Free PMC article.
-
New human rabies vaccines in the pipeline.Vaccine. 2019 Oct 3;37 Suppl 1(Suppl 1):A140-A145. doi: 10.1016/j.vaccine.2018.08.039. Epub 2018 Aug 25. Vaccine. 2019. PMID: 30153997 Free PMC article. Review.
-
Anti-HIV IgM protects against mucosal SHIV transmission.AIDS. 2018 Jul 17;32(11):F5-F13. doi: 10.1097/QAD.0000000000001857. AIDS. 2018. PMID: 29762161 Free PMC article.
-
Lymph node but not intradermal injection site macrophages are critical for germinal center formation and antibody responses to rabies vaccination.J Virol. 2015 Mar;89(5):2842-8. doi: 10.1128/JVI.03409-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540370 Free PMC article.
-
Immunoglobulin M: An Ancient Antiviral Weapon - Rediscovered.Front Immunol. 2020 Aug 11;11:1943. doi: 10.3389/fimmu.2020.01943. eCollection 2020. Front Immunol. 2020. PMID: 32849652 Free PMC article. Review.
References
-
- Bunschoten H, Dietzschold B, Claassen I, Klapmuts R, Uytdehaag F, Osterhaus A. 1990. Rabies virus cross-reactive murine T cell clones: analysis of helper and delayed-type hypersensitivity function. Viral Immunol. 3:41–53 - PubMed
-
- Baer GM. 2007. The history of rabies, p 11–19 In Jackson AC, Wunner WH. (ed), Rabies. Elsevier Science, Irvine, CA
-
- Mifune K, Takeuchi E, Napiorkowski PA, Yamada A, Sakamoto K. 1981. Essential role of T cells in the postexposure prophylaxis of rabies in mice. Microbiol. Immunol. 25:895–904 - PubMed
-
- Turner GS. 1976. Thymus dependence of rabies vaccine. J. Gen. Virol. 33:535–538 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

