α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors adopt different subunit arrangements
- PMID: 23760273
- PMCID: PMC3724652
- DOI: 10.1074/jbc.M113.469205
α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors adopt different subunit arrangements
Abstract
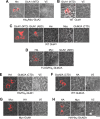
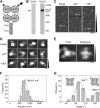
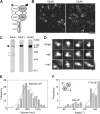
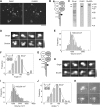
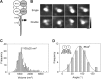
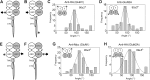
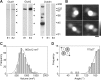
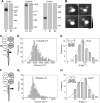
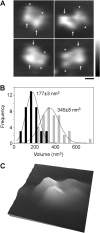
Ionotropic glutamate receptors are widely distributed in the central nervous system and play a major role in excitatory synaptic transmission. All three ionotropic glutamate subfamilies (i.e. AMPA-type, kainate-type, and NMDA-type) assemble as tetramers of four homologous subunits. There is good evidence that both heteromeric AMPA and kainate receptors have a 2:2 subunit stoichiometry and an alternating subunit arrangement. Recent studies based on presumed structural homology have indicated that NMDA receptors adopt the same arrangement. Here, we use atomic force microscopy imaging of receptor-antibody complexes to show that whereas the GluA1/GluA2 AMPA receptor assembles with an alternating (i.e. 1/2/1/2) subunit arrangement, the GluN1/GluN2A NMDA receptor adopts an adjacent (i.e. 1/1/2/2) arrangement. We conclude that the two types of ionotropic glutamate receptor are built in different ways from their constituent subunits. This surprising finding necessitates a reassessment of the assembly of these important receptors.
Keywords: Atomic Force Microscopy; Cell Surface Receptor; Glutamate Receptors, Ionotropic (AMPA, NMDA); Protein Complexes; Single-particle Analysis.
Figures
References
-
- Mansour M., Nagarajan N., Nehring R. B., Clements J. D., Rosenmund C. (2001) Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron 32, 841–853 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

