Toward a neuroimaging treatment selection biomarker for major depressive disorder
- PMID: 23760393
- PMCID: PMC4413467
- DOI: 10.1001/jamapsychiatry.2013.143
Toward a neuroimaging treatment selection biomarker for major depressive disorder
Abstract
Importance: Currently, fewer than 40% of patients treated for major depressive disorder achieve remission with initial treatment. Identification of a biological marker that might improve these odds could have significant health and economic impact.
Objective: To identify a candidate neuroimaging "treatment-specific biomarker" that predicts differential outcome to either medication or psychotherapy.
Design: Brain glucose metabolism was measured with positron emission tomography prior to treatment randomization to either escitalopram oxalate or cognitive behavior therapy for 12 weeks. Patients who did not remit on completion of their phase 1 treatment were offered enrollment in phase 2 comprising an additional 12 weeks of treatment with combination escitalopram and cognitive behavior therapy.
Setting: Mood and anxiety disorders research program at an academic medical center.
Participants: Men and women aged 18 to 60 years with currently untreated major depressive disorder.
Intervention: Randomized assignment to 12 weeks of treatment with either escitalopram oxalate (10-20 mg/d) or 16 sessions of manual-based cognitive behavior therapy.
Main outcome and measure: Remission, defined as a 17-item Hamilton depression rating scale score of 7 or less at both weeks 10 and 12, as assessed by raters blinded to treatment.
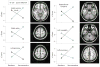
Results: Positive and negative predictors of remission were identified with a 2-way analysis of variance treatment (escitalopram or cognitive behavior therapy) × outcome (remission or nonresponse) interaction. Of 65 protocol completers, 38 patients with clear outcomes and usable positron emission tomography scans were included in the primary analysis: 12 remitters to cognitive behavior therapy, 11 remitters to escitalopram, 9 nonresponders to cognitive behavior therapy, and 6 nonresponders to escitalopram. Six limbic and cortical regions were identified, with the right anterior insula showing the most robust discriminant properties across groups (effect size = 1.43). Insula hypometabolism (relative to whole-brain mean) was associated with remission to cognitive behavior therapy and poor response to escitalopram, while insula hypermetabolism was associated with remission to escitalopram and poor response to cognitive behavior therapy.
Conclusions and relevance: If verified with prospective testing, the insula metabolism-based treatment-specific biomarker defined in this study provides the first objective marker, to our knowledge, to guide initial treatment selection for depression.
Trial registration: Registered at clinicaltrials.gov (NCT00367341).
Figures


Comment in
-
[PET reveals the best antidepressant therapy].MMW Fortschr Med. 2013 Sep 26;155(16):31. doi: 10.1007/s15006-013-2162-8. MMW Fortschr Med. 2013. PMID: 24279148 German. No abstract available.
-
Certainly not the first neuroimaging treatment selection biomarker.JAMA Psychiatry. 2014 Feb;71(2):210-1. doi: 10.1001/jamapsychiatry.2013.3303. JAMA Psychiatry. 2014. PMID: 24500631 No abstract available.
-
Significance of right anterior insula activity for mental health intervention.JAMA Psychiatry. 2014 Mar;71(3):336. doi: 10.1001/jamapsychiatry.2013.3507. JAMA Psychiatry. 2014. PMID: 24599239 No abstract available.
References
-
- Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. - PubMed
-
- Birnbaum HG, Kessler RC, Kelley D, Ben-Hamadi R, Joish VN, Greenberg PE. Employer burden of mild, moderate, and severe major depressive disorder: mental health services utilization and costs, and work performance. Depress Anxiety. 2010;27(1):78–89. - PubMed
-
- Judd LL, Akiskal HS, Zeller PJ, et al. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch Gen Psychiatry. 2000;57(4):375–380. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision.
-
- Kennedy SH, Lam RW, Parikh SV, Patten SB, Ravindran AV. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults: introduction. J Affect Disord. 2009;117(suppl 1):S1–S2. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

