Progressive multifocal leukoencephalopathy-associated mutations in the JC polyomavirus capsid disrupt lactoseries tetrasaccharide c binding
- PMID: 23760462
- PMCID: PMC3685208
- DOI: 10.1128/mBio.00247-13
Progressive multifocal leukoencephalopathy-associated mutations in the JC polyomavirus capsid disrupt lactoseries tetrasaccharide c binding
Abstract
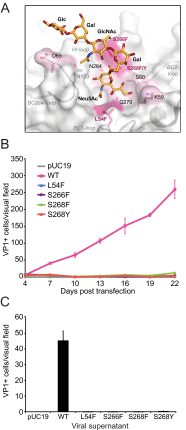
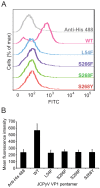
The human JC polyomavirus (JCPyV) is the causative agent of the fatal, demyelinating disease progressive multifocal leukoencephalopathy (PML). The Mad-1 prototype strain of JCPyV uses the glycan lactoseries tetrasaccharide c (LSTc) and serotonin receptor 5-HT2A to attach to and enter into host cells, respectively. Specific residues in the viral capsid protein VP1 are responsible for direct interactions with the α2,6-linked sialic acid of LSTc. Viral isolates from individuals with PML often contain mutations in the sialic acid-binding pocket of VP1 that are hypothesized to arise from positive selection. We reconstituted these mutations in the Mad-1 strain of JCPyV and found that they were not capable of growth. The mutations were then introduced into recombinant VP1 and reconstituted as pentamers in order to conduct binding studies and structural analyses. VP1 pentamers carrying PML-associated mutations were not capable of binding to permissive cells. High-resolution structure determination revealed that these pentamers are well folded but no longer bind to LSTc due to steric clashes in the sialic acid-binding site. Reconstitution of the mutations into JCPyV pseudoviruses allowed us to directly quantify the infectivity of the mutants in several cell lines. The JCPyV pseudoviruses with PML-associated mutations were not infectious, nor were they able to engage sialic acid as measured by hemagglutination of human red blood cells. These results demonstrate that viruses from PML patients with single point mutations in VP1 disrupt binding to sialic acid motifs and render these viruses noninfectious. IMPORTANCE Infection with human JC polyomavirus (JCPyV) is common and asymptomatic in healthy individuals, but during immunosuppression, JCPyV can spread from the kidney to the central nervous system (CNS) and cause a fatal, demyelinating disease, progressive multifocal leukoencephalopathy (PML). Individuals infected with HIV, those who have AIDS, or those receiving immunomodulatory therapies for autoimmune diseases are at serious risk for PML. Recent reports have demonstrated that viral isolates from PML patients often have distinct changes within the major capsid protein. Our structural-functional approach highlights that these mutations result in abolished engagement of the carbohydrate receptor motif LSTc that is necessary for infection. Viruses with PML-associated mutations are not infectious in glial cells, suggesting that they may play an alternative role in PML pathogenesis.
Figures

Similar articles
-
The Greater Affinity of JC Polyomavirus Capsid for α2,6-Linked Lactoseries Tetrasaccharide c than for Other Sialylated Glycans Is a Major Determinant of Infectivity.J Virol. 2015 Jun;89(12):6364-75. doi: 10.1128/JVI.00489-15. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855729 Free PMC article.
-
ERK Is a Critical Regulator of JC Polyomavirus Infection.J Virol. 2018 Mar 14;92(7):e01529-17. doi: 10.1128/JVI.01529-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29321332 Free PMC article.
-
JC Polyomavirus Entry by Clathrin-Mediated Endocytosis Is Driven by β-Arrestin.J Virol. 2019 Apr 3;93(8):e01948-18. doi: 10.1128/JVI.01948-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700597 Free PMC article.
-
JC polyomavirus attachment, entry, and trafficking: unlocking the keys to a fatal infection.J Neurovirol. 2015 Dec;21(6):601-13. doi: 10.1007/s13365-014-0272-4. Epub 2014 Jul 31. J Neurovirol. 2015. PMID: 25078361 Free PMC article. Review.
-
JCPyV VP1 Mutations in Progressive MultifocalLeukoencephalopathy: Altering Tropismor Mediating Immune Evasion?Viruses. 2020 Oct 12;12(10):1156. doi: 10.3390/v12101156. Viruses. 2020. PMID: 33053912 Free PMC article. Review.
Cited by
-
T cell deficiency precipitates antibody evasion and emergence of neurovirulent polyomavirus.Elife. 2022 Nov 7;11:e83030. doi: 10.7554/eLife.83030. Elife. 2022. PMID: 36341713 Free PMC article.
-
Intra-patient viral evolution in polyomavirus-related diseases.Philos Trans R Soc Lond B Biol Sci. 2019 May 27;374(1773):20180301. doi: 10.1098/rstb.2018.0301. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30955497 Free PMC article. Review.
-
Polyomavirus Wakes Up and Chooses Neurovirulence.Viruses. 2023 Oct 18;15(10):2112. doi: 10.3390/v15102112. Viruses. 2023. PMID: 37896889 Free PMC article. Review.
-
Virus-Receptor Interactions: The Key to Cellular Invasion.J Mol Biol. 2018 Aug 17;430(17):2590-2611. doi: 10.1016/j.jmb.2018.06.024. Epub 2018 Jun 18. J Mol Biol. 2018. PMID: 29924965 Free PMC article. Review.
-
Structure analysis of the major capsid proteins of human polyomaviruses 6 and 7 reveals an obstructed sialic acid binding site.J Virol. 2014 Sep;88(18):10831-9. doi: 10.1128/JVI.01084-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008942 Free PMC article.
References
-
- Kean JM, Rao S, Wang M, Garcea RL. 2009. Seroepidemiology of human polyomaviruses. PLoS Pathog. 5:e1000363 http://dx.doi.org/10.1371/journal.ppat.1000363 - PMC - PubMed
-
- Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199:837–846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

