Snail cooperates with KrasG12D to promote pancreatic fibrosis
- PMID: 23761168
- PMCID: PMC3778055
- DOI: 10.1158/1541-7786.MCR-12-0637
Snail cooperates with KrasG12D to promote pancreatic fibrosis
Abstract
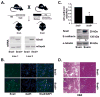
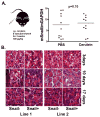
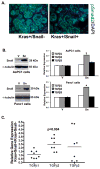
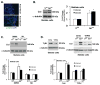
Patients with pancreatic cancer, which is characterized by an extensive collagen-rich fibrotic reaction, often present with metastases. A critical step in cancer metastasis is epithelial-to-mesenchymal transition (EMT), which can be orchestrated by the Snail family of transcription factors. To understand the role of Snail (SNAI1) in pancreatic cancer development, we generated transgenic mice expressing Snail in the pancreas. Because chronic pancreatitis can contribute to pancreatic cancer development, Snail-expressing mice were treated with cerulein to induce pancreatitis. Although significant tissue injury was observed, a minimal difference in pancreatitis was seen between control and Snail-expressing mice. However, because Kras mutation is necessary for tumor development in mouse models of pancreatic cancer, we generated mice expressing both mutant Kras(G12D) and Snail (Kras(+)/Snail(+)). Compared with control mice (Kras(+)/Snai(-)), Kras(+)/Snail(+) mice developed acinar ectasia and more advanced acinar-to-ductal metaplasia. The Kras(+)/Snail(+) mice exhibited increased fibrosis, increased phosphorylated Smad2, increased TGF-β2 expression, and activation of pancreatic stellate cells. To further understand the mechanism by which Snail promoted fibrosis, we established an in vitro model to examine the effect of Snail expression in pancreatic cancer cells on stellate cell collagen production. Snail expression in pancreatic cancer cells increased TGF-β2 levels, and conditioned media from Snail-expressing pancreatic cancer cells increased collagen production by stellate cells. Additionally, inhibiting TGF-β signaling in stellate cells attenuated the conditioned media-induced collagen production by stellate cells. Together, these results suggest that Snail contributes to pancreatic tumor development by promoting fibrotic reaction through increased TGF-β signaling.
Implications: Expression of the EMT regulator Snail in the context of mutant Kras provides new insight into pancreatic cancer progression.
©2013 AACR.
Conflict of interest statement
Figures


References
-
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

