Predictors of fracture while on treatment with oral bisphosphonates: a population-based cohort study
- PMID: 23761350
- PMCID: PMC3867340
- DOI: 10.1002/jbmr.2011
Predictors of fracture while on treatment with oral bisphosphonates: a population-based cohort study
Abstract
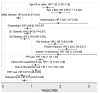
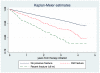
Although oral bisphosphonates (BPs) are highly effective in preventing fractures, some patients will fracture while on treatment. We identified predictors of such fractures in a population-based cohort of incident users of oral BPs. We screened the Sistema d'Informació per al Desenvolupament de l'Investigació en Atenció Primària (SIDIAP) database to identify new users of oral BPs in 2006-2007. SIDIAP includes pharmacy invoice data and primary care electronic medical records for a representative 5 million people in Catalonia (Spain). Exclusion criteria were the following: Paget disease; <40 years of age; and any antiosteoporosis treatment in the previous year. A priori defined risk factors included age, gender, body mass index, vitamin D deficiency, smoking, alcohol drinking, preexisting comorbidities, and medications. Fractures were considered if they appeared at least 6 months after treatment initiation. "Fractures while on treatment" were defined as those occurring among participants persisting for at least 6 months and with an overall high compliance (medication possession ratio ≥80%). Fine and Gray survival models accounting for competing risk with therapy discontinuation were fitted to identify key predictors. Only 7449 of 21,385 (34.8%) participants completed >6 months of therapy. Incidence of fracture while on treatment was 3.4/100 person-years (95% confidence interval [CI], 3.1-3.7). Predictors of these among patients persisting and adhering to treatment included: older age (subhazard ratio [SHR] for 60 to <80 years, 2.18 [95% CI, 1.70-2.80]; for ≥80 years, 2.5 [95% CI, 1.82-3.43]); previous fracture (1.75 [95% CI, 1.39-2.20] and 2.49 [95% CI, 1.98-3.13], in the last 6 months and longer, respectively); underweight, 2.11 (95% CI, 1.14-3.92); inflammatory arthritis, 1.46 (95% CI, 1.02-2.10); use of proton pump inhibitors (PPIs), 1.22 (95% CI, 1.02-1.46); and vitamin D deficiency, 2.69 (95% CI, 1.27-5.72). Even among high compliers, 3.4% of oral BP users will fracture every year. Older age, underweight, vitamin D deficiency, PPI use, previous fracture, and inflammatory arthritides increase risk. Monitoring strategies and/or alternative therapies should be considered for these patients.
Keywords: BISPHOSPHONATES; BONE; EPIDEMIOLOGY; FRACTURES.
© 2014 American Society for Bone and Mineral Research.
Figures



Comment in
-
Should we be satisfied with the available anti-fracture therapies?Climacteric. 2013 Dec;16(6):719-20. Climacteric. 2013. PMID: 24383091 No abstract available.
Similar articles
-
Incidence and Predictors of Multiple Fractures Despite High Adherence to Oral Bisphosphonates: A Binational Population-Based Cohort Study.J Bone Miner Res. 2016 Jan;31(1):234-44. doi: 10.1002/jbmr.2595. J Bone Miner Res. 2016. PMID: 26174968 Clinical Trial.
-
The association between fracture site and obesity in men: a population-based cohort study.J Bone Miner Res. 2013 Aug;28(8):1771-7. doi: 10.1002/jbmr.1878. J Bone Miner Res. 2013. PMID: 23371035
-
Incident type 2 diabetes and hip fracture risk: a population-based matched cohort study.Osteoporos Int. 2015 Feb;26(2):827-33. doi: 10.1007/s00198-014-2986-9. Epub 2014 Dec 9. Osteoporos Int. 2015. PMID: 25488807
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Screening for the primary prevention of fragility fractures among adults aged 40 years and older in primary care: systematic reviews of the effects and acceptability of screening and treatment, and the accuracy of risk prediction tools.Syst Rev. 2023 Mar 21;12(1):51. doi: 10.1186/s13643-023-02181-w. Syst Rev. 2023. PMID: 36945065 Free PMC article.
Cited by
-
Predictors of re-fracture amongst patients managed within a secondary fracture prevention program: a 7-year prospective study.Osteoporos Int. 2015 Feb;26(2):543-51. doi: 10.1007/s00198-014-2880-5. Epub 2014 Sep 5. Osteoporos Int. 2015. PMID: 25189427
-
Acid-Suppressive Therapy and Risk of Infections: Pros and Cons.Clin Drug Investig. 2017 Jul;37(7):587-624. doi: 10.1007/s40261-017-0519-y. Clin Drug Investig. 2017. PMID: 28361440 Review.
-
Predictors of Hip Fracture Despite Treatment with Bisphosphonates among Frail Older Adults.J Am Geriatr Soc. 2020 Feb;68(2):256-260. doi: 10.1111/jgs.16176. Epub 2019 Oct 3. J Am Geriatr Soc. 2020. PMID: 31580488 Free PMC article.
-
Identifying patients on osteoporosis treatment who are at highest risk of fractures.Bonekey Rep. 2013 Nov 27;2:471. doi: 10.1038/bonekey.2013.205. eCollection 2013. Bonekey Rep. 2013. PMID: 24422162 Free PMC article. No abstract available.
-
Bone fracture and the interaction between bisphosphonates and proton pump inhibitors: a meta-analysis.Int J Clin Exp Med. 2015 Apr 15;8(4):4899-910. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26131063 Free PMC article.
References
-
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. - PubMed
-
- Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR, FIT Research Group Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab. 2000;85(11):4118–24. - PubMed
-
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD, Vertebral Efficacy With Risedronate Therapy (VERT) Study Group Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282(14):1344–52. - PubMed
-
- Sheehy O, Kindundu CM, Barbeau M, LeLorier J. Differences in persistence among different weekly oral bisphosphonate medications. Osteoporos Int. 2009;20(8):1369–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

