Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration
- PMID: 23761625
- PMCID: PMC3761151
- DOI: 10.1152/ajpcell.00051.2013
Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration
Abstract
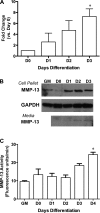
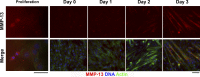
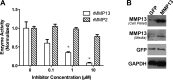
Efficient skeletal muscle repair and regeneration require coordinated remodeling of the extracellular matrix (ECM). Previous reports have indicated that matrix metalloproteinases (MMPs) play the pivotal role in ECM remodeling during muscle regeneration. The goal of the current study was to determine if the interstitial collagenase MMP-13 was involved in the muscle repair process. Using intramuscular cardiotoxin injections to induce acute muscle injury, we found that MMP-13 expression and activity transiently increased during the regeneration process. In addition, in muscles from mdx mice, which exhibit chronic injury, MMP-13 expression and protein levels were elevated. In differentiating C2C12 cells, a murine myoblast cell line, Mmp13 expression was most pronounced after myoblast fusion and during myotube formation. Using pharmacological inhibition of MMP-13 to test whether MMP-13 activity is necessary for the proliferation, differentiation, migration, and fusion of C2C12 cells, we found a dramatic blockade of myoblast migration, as well as a delay in differentiation. In contrast, C2C12 cells with stable overexpression of MMP-13 showed enhanced migration, without affecting myoblast maturation. Taken together, these results support a primary role for MMP-13 in myoblast migration that leads to secondary effects on differentiation.
Keywords: collagenase; matrix metalloproteinase; muscle repair; myoblast maturation.
Figures





Similar articles
-
Matrix Metalloproteinase 13 from Satellite Cells is Required for Efficient Muscle Growth and Regeneration.Cell Physiol Biochem. 2020 Apr 11;54(3):333-353. doi: 10.33594/000000223. Cell Physiol Biochem. 2020. PMID: 32275813 Free PMC article.
-
Matrix metalloproteinase-1 promotes muscle cell migration and differentiation.Am J Pathol. 2009 Feb;174(2):541-9. doi: 10.2353/ajpath.2009.080509. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147819 Free PMC article.
-
Decrease of MMP-9 activity improves soleus muscle regeneration.Tissue Eng Part A. 2012 Jun;18(11-12):1183-92. doi: 10.1089/ten.TEA.2011.0459. Epub 2012 Apr 20. Tissue Eng Part A. 2012. PMID: 22429194 Free PMC article.
-
MMP-14 in skeletal muscle repair.J Muscle Res Cell Motil. 2015 Jun;36(3):215-25. doi: 10.1007/s10974-015-9414-4. Epub 2015 May 30. J Muscle Res Cell Motil. 2015. PMID: 26025393 Review.
-
MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix.J Appl Physiol (1985). 2013 Sep;115(6):884-91. doi: 10.1152/japplphysiol.00137.2013. Epub 2013 May 2. J Appl Physiol (1985). 2013. PMID: 23640595 Free PMC article. Review.
Cited by
-
Transcriptome Profiling of Osteoblasts in a Medaka (Oryzias latipes) Osteoporosis Model Identifies Mmp13b as Crucial for Osteoclast Activation.Front Cell Dev Biol. 2022 Feb 21;10:775512. doi: 10.3389/fcell.2022.775512. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35281094 Free PMC article.
-
Mature IGF-I excels in promoting functional muscle recovery from disuse atrophy compared with pro-IGF-IA.J Appl Physiol (1985). 2014 Apr 1;116(7):797-806. doi: 10.1152/japplphysiol.00955.2013. Epub 2013 Dec 26. J Appl Physiol (1985). 2014. PMID: 24371018 Free PMC article.
-
Acute and Delayed Effects of Time-Matched Very Short "All Out" Efforts in Concentric vs. Eccentric Cycling.Int J Environ Res Public Health. 2021 Jul 28;18(15):7968. doi: 10.3390/ijerph18157968. Int J Environ Res Public Health. 2021. PMID: 34360257 Free PMC article.
-
Tumor Necrosis Factor-Alpha Modulates Expression of Genes Involved in Cytokines and Chemokine Pathways in Proliferative Myoblast Cells.Cells. 2024 Jul 8;13(13):1161. doi: 10.3390/cells13131161. Cells. 2024. PMID: 38995013 Free PMC article.
-
Possible Involvement of CSPG4 in Promoting Endothelial Cell Migration and Contributing to Angiogenesis during Skeletal Muscle Regeneration and Development in the Rat.Anim Sci J. 2025 Jan-Dec;96(1):e70097. doi: 10.1111/asj.70097. Anim Sci J. 2025. PMID: 40769519 Free PMC article.
References
-
- Bani C, Lagrota-Candido J, Pinheiro DF, Leite PE, Salimena MC, Henriques-Pons A, Quirico-Santos T. Pattern of metalloprotease activity and myofiber regeneration in skeletal muscles of mdx mice. Muscle Nerve 37: 583–592, 2008 - PubMed
-
- Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1–14, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

