Chenodeoxycholic acid stimulates Cl(-) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells
- PMID: 23761628
- PMCID: PMC3891218
- DOI: 10.1152/ajpcell.00416.2012
Chenodeoxycholic acid stimulates Cl(-) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells
Abstract
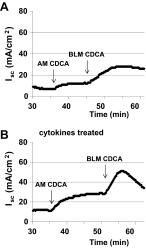
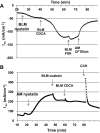
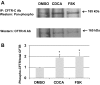
High levels of chenodeoxycholic acid (CDCA) and deoxycholic acid stimulate Cl(-) secretion in mammalian colonic epithelia. While different second messengers have been implicated in this action, the specific signaling pathway has not been fully delineated. Using human colon carcinoma T84 cells, we elucidated this cascade assessing Cl(-) transport by measuring I(-) efflux and short-circuit current (Isc). CDCA (500 μM) rapidly increases I(-) efflux, and we confirmed by Isc that it elicits a larger response when added to the basolateral vs. apical surface. However, preincubation with cytokines increases the monolayer responsiveness to apical addition by 55%. Nystatin permeabilization studies demonstrate that CDCA stimulates an eletrogenic apical Cl(-) but not a basolateral K(+) current. Furthermore, CDCA-induced Isc was inhibited (≥67%) by bumetanide, BaCl2, and the cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor CFTRinh-172. CDCA-stimulated Isc was decreased 43% by the adenylate cyclase inhibitor MDL12330A and CDCA increases intracellular cAMP concentration. The protein kinase A inhibitor H89 and the microtubule disrupting agent nocodazole, respectively, cause 94 and 47% reductions in CDCA-stimulated Isc. Immunoprecipitation with CFTR antibodies, followed by sequential immunoblotting with Pan-phospho and CFTR antibodies, shows that CDCA increases CFTR phosphorylation by approximately twofold. The rapidity and side specificity of the response to CDCA imply a membrane-mediated process. While CDCA effects are not blocked by the muscarinic receptor antagonist atropine, T84 cells possess transcript and protein for the bile acid G protein-coupled receptor TGR5. These results demonstrate for the first time that CDCA activates CFTR via a cAMP-PKA pathway involving microtubules and imply that this occurs via a basolateral membrane receptor.
Keywords: CDCA; CFTR; T84 cells; cAMP signaling.
Figures






References
-
- Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res 24: 1803– 1823, 2007 - PubMed
-
- Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339– 351, 2011 - PubMed
-
- Barrett KE. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol Cell Physiol 265: C859– C868, 1993 - PubMed
-
- Bijvelds MJ, Jorna H, Verkade HJ, Bot AG, Hofmann F, Agellon LB, Sinaasappel M, de Jonge HR. Activation of CFTR by ASBT-mediated bile salt absorption. Am J Physiol Gastrointest Liver Physiol 289: G870– G879, 2005 - PubMed
-
- Boonkaewwan C, Ao M, Toskulkao C, Rao MC. Specific immunomodulatory and secretory activities of stevioside and steviol in intestinal cells. J Agric Food Chem 56: 3777– 3784, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

