BMP signaling in telencephalic neural cell specification and maturation
- PMID: 23761735
- PMCID: PMC3671186
- DOI: 10.3389/fncel.2013.00087
BMP signaling in telencephalic neural cell specification and maturation
Abstract
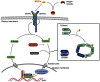
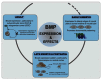
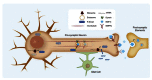
Bone morphogenetic proteins (BMPs) make up a family of morphogens that are critical for patterning, development, and function of the central and peripheral nervous system. Their effects on neural cells are pleiotropic and highly dynamic depending on the stage of development and the local niche. Neural cells display a broad expression profile of BMP ligands, receptors, and transducer molecules. Moreover, interactions of BMP signaling with other incoming morphogens and signaling pathways are crucial for most of these processes. The key role of BMP signaling suggests that it includes many regulatory mechanisms that restrict BMP activity both temporally and spatially. BMPs affect neural cell fate specification in a dynamic fashion. Initially they inhibit proliferation of neural precursors and promote the first steps in neuronal differentiation. Later on, BMP signaling effects switch from neuronal induction to promotion of astroglial identity and inhibition of neuronal or oligodendroglial lineage commitment. Furthermore, in postmitotic cells, BMPs regulate cell survival and death, to modulate neuronal subtype specification, promote dendritic and axonal growth and induce synapse formation and stabilization. In this review, we examine the canonical and non-canonical mechanisms of BMP signal transduction. Moreover, we focus on the specific role of BMPs in the nervous system including their ability to regulate neural stem cell proliferation, self-renewal, lineage specification, and neuronal function.
Keywords: BMP; morphogen; neural development; neural differentiation; signal transduction; synaptogenesis.
Figures


References
-
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhaes T. R., Goodman C. S. (2002). wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33 545–558.doi: 10.1016/S0896-6273(02)00589-5 - PubMed
-
- Anderson R. M., Lawrence A. R., Stottmann R. W., Bachiller D., Klingensmith J. (2002). Chordin and noggin promote organizing centers of forebrain development in the mouse. Development 129 4975–4987 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

