AZ-4217: a high potency BACE inhibitor displaying acute central efficacy in different in vivo models and reduced amyloid deposition in Tg2576 mice
- PMID: 23761903
- PMCID: PMC6618391
- DOI: 10.1523/JNEUROSCI.1165-13.2013
AZ-4217: a high potency BACE inhibitor displaying acute central efficacy in different in vivo models and reduced amyloid deposition in Tg2576 mice
Abstract
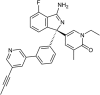
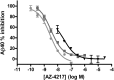
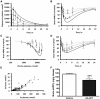
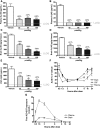
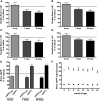
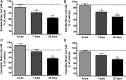
Aβ, the product of APP (amyloid precursor protein), has been implicated in the pathophysiology of Alzheimer's disease (AD). β-Site APP cleaving enzyme1 (BACE1) is the enzyme initiating the processing of the APP to Aβ peptides. Small molecule BACE1 inhibitors are expected to decrease Aβ-peptide generation and thereby reduce amyloid plaque formation in the brain, a neuropathological hallmark of AD. BACE1 inhibition thus addresses a key mechanism in AD and its potential as a therapeutic target is currently being addressed in clinical studies. Here, we report the discovery and the pharmacokinetic and pharmacodynamic properties of BACE1 inhibitor AZ-4217, a high potency compound (IC50 160 pM in human SH-SY5Y cells) with an excellent in vivo efficacy. Central efficacy of BACE1 inhibition was observed after a single dose in C57BL/6 mice, guinea pigs, and in an APP transgenic mouse model of cerebral amyloidosis (Tg2576). Furthermore, we demonstrate that in a 1 month treatment paradigm BACE1 inhibition of Aβ production does lower amyloid deposition in 12-month-old Tg2576 mice. These results strongly support BACE1 inhibition as concretely impacting amyloid deposition and therefore potentially an important approach for therapeutic intervention in AD.
Figures
Similar articles
-
Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis.Neuron. 2008 Dec 26;60(6):988-1009. doi: 10.1016/j.neuron.2008.10.047. Neuron. 2008. PMID: 19109907 Free PMC article.
-
Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor.J Neurosci. 2011 Nov 16;31(46):16507-16. doi: 10.1523/JNEUROSCI.3647-11.2011. J Neurosci. 2011. PMID: 22090477 Free PMC article. Clinical Trial.
-
Swedish mutant APP-based BACE1 binding site peptide reduces APP β-cleavage and cerebral Aβ levels in Alzheimer's mice.Sci Rep. 2015 Jun 19;5:11322. doi: 10.1038/srep11322. Sci Rep. 2015. PMID: 26091071 Free PMC article.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
-
Synthetic nucleic acids delivered by exosomes: a potential therapeutic for generelated metabolic brain diseases.Metab Brain Dis. 2013 Dec;28(4):551-62. doi: 10.1007/s11011-013-9434-y. Metab Brain Dis. 2013. PMID: 24022398 Review.
-
Inflammatory Eicosanoids Increase Amyloid Precursor Protein Expression via Activation of Multiple Neuronal Receptors.Sci Rep. 2015 Dec 17;5:18286. doi: 10.1038/srep18286. Sci Rep. 2015. PMID: 26672557 Free PMC article.
-
Differential transgene expression patterns in Alzheimer mouse models revealed by novel human amyloid precursor protein-specific antibodies.Aging Cell. 2016 Oct;15(5):953-63. doi: 10.1111/acel.12508. Epub 2016 Jul 29. Aging Cell. 2016. PMID: 27470171 Free PMC article.
-
Expanding the Repertoire of Biomarkers for Alzheimer's Disease: Targeted and Non-targeted Approaches.Front Neurol. 2015 Dec 16;6:256. doi: 10.3389/fneur.2015.00256. eCollection 2015. Front Neurol. 2015. PMID: 26733934 Free PMC article. Review.
-
Inhibiting BACE1 to reverse synaptic dysfunctions in Alzheimer's disease.Neurosci Biobehav Rev. 2016 Jun;65:326-40. doi: 10.1016/j.neubiorev.2016.03.025. Epub 2016 Apr 1. Neurosci Biobehav Rev. 2016. PMID: 27044452 Free PMC article. Review.
References
-
- Cai J, Qi X, Kociok N, Skosyrski S, Emilio A, Ruan Q, Han S, Liu L, Chen Z, Bowes Rickman C, Golde T, Grant MB, Saftig P, Serneels L, de strooper B, Joussen AM, Boulton ME. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol Med. 2012;4:980–991. doi: 10.1002/emmm.201101084. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
