Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork
- PMID: 23762018
- PMCID: PMC3676292
- DOI: 10.1371/journal.pbio.1001577
Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork
Abstract
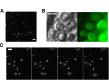
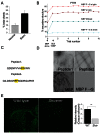
Rapid conduction of nerve impulses requires coating of axons by myelin. To function as an electrical insulator, myelin is generated as a tightly packed, lipid-rich multilayered membrane sheath. Knowledge about the mechanisms that govern myelin membrane biogenesis is required to understand myelin disassembly as it occurs in diseases such as multiple sclerosis. Here, we show that myelin basic protein drives myelin biogenesis using weak forces arising from its inherent capacity to phase separate. The association of myelin basic protein molecules to the inner leaflet of the membrane bilayer induces a phase transition into a cohesive mesh-like protein network. The formation of this protein network shares features with amyloid fibril formation. The process is driven by phenylalanine-mediated hydrophobic and amyloid-like interactions that provide the molecular basis for protein extrusion and myelin membrane zippering. These findings uncover a physicochemical mechanism of how a cytosolic protein regulates the morphology of a complex membrane architecture. These results provide a key mechanism in myelin membrane biogenesis with implications for disabling demyelinating diseases of the central nervous system.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
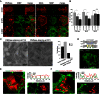
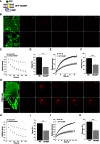
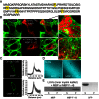
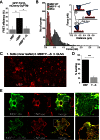
References
-
- Hyman AA, Simons K (2012) Beyond oil and water-phase transitions in cells. Science 337: 1047–1049. - PubMed
-
- Weber Stephanie C, Brangwynne Clifford P (2012) Getting RNA and protein in phase. Cell 149: 1188–1191. - PubMed
-
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, et al. (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324: 1729–1732. - PubMed
-
- Emery B (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330: 779–782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

