Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection
- PMID: 23762029
- PMCID: PMC3675149
- DOI: 10.1371/journal.ppat.1003407
Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection
Abstract
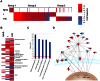
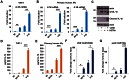
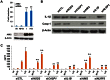
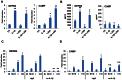
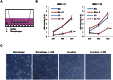
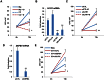
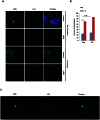
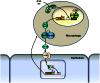
Although vitamin D deficiency is a common feature among patients presenting with active tuberculosis, the full scope of vitamin D action during Mycobacterium tuberculosis (Mtb) infection is poorly understood. As macrophages are the primary site of Mtb infection and are sites of vitamin D signaling, we have used these cells to understand the molecular mechanisms underlying modulation of the immune response by the hormonal form of vitamin D, 1,25-dihydroxyvitamin D (1,25D). We found that the virulent Mtb strain H37Rv elicits a broad host transcriptional response. Transcriptome profiling also revealed that the profile of target genes regulated by 1,25D is substantially altered by infection, and that 1,25D generally boosts infection-stimulated cytokine/chemokine responses. We further focused on the role of 1,25D- and infection-induced interleukin 1β (IL-1β) expression in response to infection. 1,25D enhanced IL-1β expression via a direct transcriptional mechanism. Secretion of IL-1β from infected cells required the NLRP3/caspase-1 inflammasome. The impact of IL-1β production was investigated in a novel model wherein infected macrophages were co-cultured with primary human small airway epithelial cells. Co-culture significantly prolonged survival of infected macrophages, and 1,25D/infection-induced IL-1β secretion from macrophages reduced mycobacterial burden by stimulating the anti-mycobacterial capacity of co-cultured lung epithelial cells. These effects were independent of 1,25D-stimulated autophagy in macrophages but dependent upon epithelial IL1R1 signaling and IL-1β-driven epithelial production of the antimicrobial peptide DEFB4/HBD2. These data provide evidence that the anti-microbial actions of vitamin D extend beyond the macrophage by modulating paracrine signaling, reinforcing its role in innate immune regulation in humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Dye C (2006) Global epidemiology of tuberculosis. The Lancet 367: 938–940. - PubMed
-
- Keeler E, Perkins MD, Small P, Hanson C, Reed S, et al. (2006) Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 444 Suppl 1: 49–57. - PubMed
-
- Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL (2008) Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 8: 142–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

