Novel biodegradable porous scaffold applied to skin regeneration
- PMID: 23762223
- PMCID: PMC3677897
- DOI: 10.1371/journal.pone.0056330
Novel biodegradable porous scaffold applied to skin regeneration
Erratum in
- PLoS One. 2013;8(11). doi:10.1371/annotation/4d5ef06d-b800-4d0c-b809-d3cb7a5d00c6. Wang, Zhao-Ren [corrected to Wang, Chau-Zen]
Abstract
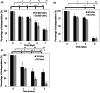
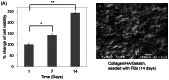
Skin wound healing is an important lifesaving issue for massive lesions. A novel porous scaffold with collagen, hyaluronic acid and gelatin was developed for skin wound repair. The swelling ratio of this developed scaffold was assayed by water absorption capacity and showed a value of over 20 g water/g dried scaffold. The scaffold was then degraded in time- and dose-dependent manners by three enzymes: lysozyme, hyaluronidase and collagenase I. The average pore diameter of the scaffold was 132.5±8.4 µm measured from SEM images. With human skin cells growing for 7 days, the SEM images showed surface fractures on the scaffold due to enzymatic digestion, indicating the biodegradable properties of this scaffold. To simulate skin distribution, the human epidermal keratinocytes, melanocytes and dermal fibroblasts were seeded on the porous scaffold and the cross-section immunofluorescent staining demonstrated normal human skin layer distributions. The collagen amount was also quantified after skin cells seeding and presented an amount 50% higher than those seeded on culture wells. The in vivo histological results showed that the scaffold ameliorated wound healing, including decreasing neutrophil infiltrates and thickening newly generated skin compared to the group without treatments.
Conflict of interest statement
Figures







References
-
- Pomahac B, Svensjo T, Yao F, Brown H, Eriksson E (1998) Tissue engineering of skin. Crit Rev Oral Biol Med 9: 333–344. - PubMed
-
- Boyce ST (2001) Design principles for composition and performance of cultured skin substitutes. Burns 27: 523–533. - PubMed
-
- Ma L, Gao C, Mao Z, Zhou J, Shen J, et al. (2003) Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 24: 4833–4841. - PubMed
-
- Schulz JT 3rd, Tompkins RG, Burke JF (2000) Artificial skin. Annu Rev Med 51: 231–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

