The elongation complex components BRD4 and MLLT3/AF9 are transcriptional coactivators of nuclear retinoid receptors
- PMID: 23762261
- PMCID: PMC3677938
- DOI: 10.1371/journal.pone.0064880
The elongation complex components BRD4 and MLLT3/AF9 are transcriptional coactivators of nuclear retinoid receptors
Abstract
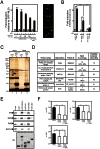
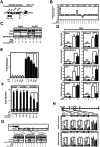
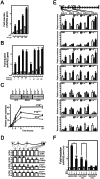
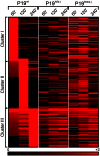
Nuclear all-trans retinoic acid receptors (RARs) initiate early transcriptional events which engage pluripotent cells to differentiate into specific lineages. RAR-controlled transactivation depends mostly on agonist-induced structural transitions in RAR C-terminus (AF-2), thus bridging coactivators or corepressors to chromatin, hence controlling preinitiation complex assembly. However, the contribution of other domains of RAR to its overall transcriptional activity remains poorly defined. A proteomic characterization of nuclear proteins interacting with RAR regions distinct from the AF-2 revealed unsuspected functional properties of the RAR N-terminus. Indeed, mass spectrometry fingerprinting identified the Bromodomain-containing protein 4 (BRD4) and ALL1-fused gene from chromosome 9 (AF9/MLLT3), known to associate with and regulates the activity of Positive Transcription Elongation Factor b (P-TEFb), as novel RAR coactivators. In addition to promoter sequences, RAR binds to genomic, transcribed regions of retinoid-regulated genes, in association with RNA polymerase II and as a function of P-TEFb activity. Knockdown of either AF9 or BRD4 expression affected differentially the neural differentiation of stem cell-like P19 cells. Clusters of retinoid-regulated genes were selectively dependent on BRD4 and/or AF9 expression, which correlated with RAR association to transcribed regions. Thus RAR establishes physical and functional links with components of the elongation complex, enabling the rapid retinoid-induced induction of genes required for neuronal differentiation. Our data thereby extends the previously known RAR interactome from classical transcriptional modulators to components of the elongation machinery, and unravel a functional role of RAR in transcriptional elongation.
Conflict of interest statement
Figures



References
-
- Samarut E, Rochette-Egly C (2012) Nuclear retinoic acid receptors: conductors of the retinoic acid symphony during development. Mol Cell Endocrinol 348: 348–360. - PubMed
-
- Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, et al. (2006) A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312: 1798–1802. - PubMed
-
- Pavri R, Lewis B, Kim TK, Dilworth FJ, Erdjument-Bromage H, et al. (2005) PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol Cell 18: 83–96. - PubMed
-
- Lefebvre B, Brand C, Flajollet S, Lefebvre P (2006) Down-Regulation of the Tumor Suppressor Gene Retinoic Acid Receptor {beta}2 through the Phosphoinositide 3-Kinase/Akt Signaling Pathway. Mol Endocrinol 20: 2109–2121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

