3-oxoacyl-ACP reductase from Schistosoma japonicum: integrated in silico-in vitro strategy for discovering antischistosomal lead compounds
- PMID: 23762275
- PMCID: PMC3676400
- DOI: 10.1371/journal.pone.0064984
3-oxoacyl-ACP reductase from Schistosoma japonicum: integrated in silico-in vitro strategy for discovering antischistosomal lead compounds
Abstract
Background: Schistosomiasis is a disease caused by parasitic worms and more than 200 million people are infected worldwide. The emergence of resistance to the most commonly used drug, praziquantel (PZQ), makes the development of novel drugs an urgent task. 3-oxoacyl-ACP reductase (OAR), a key enzyme involved in the fatty acid synthesis pathway, has been identified as a potential drug target against many pathogenic organisms. However, no research on Schistosoma japonicum OAR (SjOAR) has been reported. The characterization of the SjOAR protein will provide new strategies for screening antischistosomal drugs that target SjOAR.
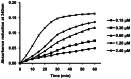
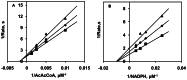
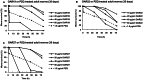
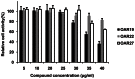
Methodology/principal findings: After cloning the SjOAR gene, recombinant SjOAR protein was purified and assayed for enzymatic activity. The tertiary structure of SjOAR was obtained by homology modeling and 27 inhibitor candidates were identified from 14,400 compounds through molecular docking based on the structure. All of these compounds were confirmed to be able to bind to the SjOAR protein by BIAcore analysis. Two compounds exhibited strong antischistosomal activity and inhibitory effects on the enzymatic activity of SjOAR. In contrast, these two compounds showed relatively low toxicity towards host cells.
Conclusions/significance: The work presented here shows the feasibility of isolation of new antischistosomal compounds using a combination of virtual screening and experimental validation. Based on this strategy, we successfully identified 2 compounds that target SjOAR with strong antischistosomal activity but relatively low cytotoxicity to host cells.
Conflict of interest statement
Figures




Similar articles
-
Aldose reductase from Schistosoma japonicum: crystallization and structure-based inhibitor screening for discovering antischistosomal lead compounds.Parasit Vectors. 2013 Jun 5;6:162. doi: 10.1186/1756-3305-6-162. Parasit Vectors. 2013. PMID: 23734964 Free PMC article.
-
Molecular docking to explore the possible binding mode of potential inhibitors of thioredoxin glutathione reductase.Mol Med Rep. 2015 Oct;12(4):5787-95. doi: 10.3892/mmr.2015.4119. Epub 2015 Jul 27. Mol Med Rep. 2015. PMID: 26239395 Free PMC article.
-
Targeting thioredoxin glutathione reductase as a potential antischistosomal drug target.Mol Biochem Parasitol. 2018 Oct;225:94-102. doi: 10.1016/j.molbiopara.2018.09.004. Epub 2018 Oct 4. Mol Biochem Parasitol. 2018. PMID: 30291946 Review.
-
Thioredoxin glutathione reductase as a novel drug target: evidence from Schistosoma japonicum.PLoS One. 2012;7(2):e31456. doi: 10.1371/journal.pone.0031456. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22384025 Free PMC article.
-
Research and development of antischistosomal drugs in the People's Republic of China a 60-year review.Adv Parasitol. 2010;73:231-95. doi: 10.1016/S0065-308X(10)73009-8. Adv Parasitol. 2010. PMID: 20627145 Review.
Cited by
-
High Throughput and Computational Repurposing for Neglected Diseases.Pharm Res. 2018 Dec 17;36(2):27. doi: 10.1007/s11095-018-2558-3. Pharm Res. 2018. PMID: 30560386 Free PMC article. Review.
-
Immunoproteomics and Surfaceomics of the Adult Tapeworm Hymenolepis diminuta.Front Immunol. 2018 Nov 12;9:2487. doi: 10.3389/fimmu.2018.02487. eCollection 2018. Front Immunol. 2018. PMID: 30483248 Free PMC article.
-
Modern approaches to accelerate discovery of new antischistosomal drugs.Expert Opin Drug Discov. 2016 Jun;11(6):557-67. doi: 10.1080/17460441.2016.1178230. Epub 2016 May 3. Expert Opin Drug Discov. 2016. PMID: 27073973 Free PMC article. Review.
-
Schistosomiasis Drug Discovery in the Era of Automation and Artificial Intelligence.Front Immunol. 2021 May 31;12:642383. doi: 10.3389/fimmu.2021.642383. eCollection 2021. Front Immunol. 2021. PMID: 34135888 Free PMC article. Review.
-
Borrelia burgdorferi infection modifies protein content in saliva of Ixodes scapularis nymphs.BMC Genomics. 2021 Mar 4;22(1):152. doi: 10.1186/s12864-021-07429-0. BMC Genomics. 2021. PMID: 33663385 Free PMC article.
References
-
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6: 411–425. - PubMed
-
- Chen HC, Xie SY, Zeng XJ, Huang XB, Wang TP, et al. (2011) [Current endemic situation and control strategy of schistosomiasis in lake and marshland regions in China]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 23: 5–9. - PubMed
-
- Chen MG (2005) Use of praziquantel for clinical treatment and morbidity control of schistosomiasis japonica in China: a review of 30 years' experience. Acta Trop 96: 168–176. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

