Biochemical and functional analysis of Drosophila-sciara chimeric sex-lethal proteins
- PMID: 23762307
- PMCID: PMC3677924
- DOI: 10.1371/journal.pone.0065171
Biochemical and functional analysis of Drosophila-sciara chimeric sex-lethal proteins
Abstract
Background: The Drosophila SXL protein controls sex determination and dosage compensation. It is a sex-specific factor controlling splicing of its own Sxl pre-mRNA (auto-regulation), tra pre-mRNA (sex determination) and msl-2 pre-mRNA plus translation of msl-2 mRNA (dosage compensation). Outside the drosophilids, the same SXL protein has been found in both sexes so that, in the non-drosophilids, SXL does not appear to play the key discriminating role in sex determination and dosage compensation that it plays in Drosophila. Comparison of SXL proteins revealed that its spatial organisation is conserved, with the RNA-binding domains being highly conserved, whereas the N- and C-terminal domains showing significant variation. This manuscript focuses on the evolution of the SXL protein itself and not on regulation of its expression.
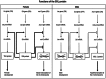
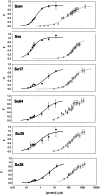
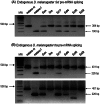
Methodology: Drosophila-Sciara chimeric SXL proteins were produced. Sciara SXL represents the non-sex-specific function of ancient SXL in the non-drosophilids from which presumably Drosophila SXL evolved. Two questions were addressed. Did the Drosophila SXL protein have affected their functions when their N- and C-terminal domains were replaced by the corresponding ones of Sciara? Did the Sciara SXL protein acquire Drosophila sex-specific functions when the Drosophila N- and C-terminal domains replaced those of Sciara? The chimeric SXL proteins were analysed in vitro to study their binding affinity and cooperative properties, and in vivo to analyse their effect on sex determination and dosage compensation by producing Drosophila flies that were transgenic for the chimeric SXL proteins.
Conclusions: The sex-specific properties of extant Drosophila SXL protein depend on its global structure rather than on a specific domain. This implies that the modifications, mainly in the N- and C-terminal domains, that occurred in the SXL protein during its evolution within the drosophilid lineage represent co-evolutionary changes that determine the appropriate folding of SXL to carry out its sex-specific functions.
Conflict of interest statement
Figures
References
-
- Bachiller D, Sánchez L (1991) Production of XO clones in XX females of Drosophila . Genet Res 57: 23–28. - PubMed
-
- Bell LR, Horabin JI, Schedl P, Cline TW (1991) Positive autoregulation of Sex-lethal by alternative splicing maintains the female determined state in Drosophila. . Cell 65: 229–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

