The role of the e3 ligase cbl-B in murine dendritic cells
- PMID: 23762309
- PMCID: PMC3675148
- DOI: 10.1371/journal.pone.0065178
The role of the e3 ligase cbl-B in murine dendritic cells
Abstract
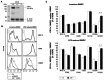
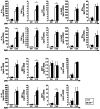
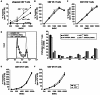
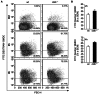
Dendritic cells (DCs) are potent antigen-presenting cells with a promising potential in cancer immunotherapy. Cbl proteins are E3 ubiquitin ligases and have been implicated in regulating the functional activity of various immune cells. As an example, c-Cbl negatively affects DC activation. We here describe that another member of the Cbl-protein family (i.e. Cbl-b) is highly expressed in murine bone-marrow-derived DCs (BMDCs). Differentiation of cblb-/- bone marrow mononuclear cells into classical BMDCs is unaltered, except enhanced induction of DEC-205 (CD205) expression. When tested in mixed-lymphocyte reaction (MLR), cblb-/- BMDCs exhibit increased allo-stimulatory capacity in vitro. BMDCs were next in vitro stimulated by various toll like receptor (TLR)-agonists (LPS, Poly(I:C), CpG) and exposed to FITC-labeled dextran. Upon TLR-stimulation, cblb-/- BMDCs produce higher levels of proinflammatory cytokines (IL-1α, IL-6 and TNF-α) and exhibit a slightly higher level of FITC-dextran uptake. To further characterize the functional significance of cblb-/- BMDCs we tested them in antigen-specific T cell responses against ovalbumin (OVA) protein and peptides, activating either CD8(+) OT-I or CD4(+) OT-II transgenic T cells. However, cblb-/- BMDCs are equally effective in inducing antigen-specific T cell responses when compared to wildtype BMDCs both in vitro and in vivo. The migratory capacity into lymph nodes during inflammation was similarly not affected by the absence of Cbl-b. In line with these observations, cblb-/- peptide-pulsed BMDCs are equally effective vaccines against OVA-expressing B16 tumors in vivo when compared to wildtype BMDCs. We conclude that in contrast to c-Cbl, Cbl-b plays only a limited role in the induction of Ag-specific T cell responses by murine BMDCs in vitro and in vivo.
Conflict of interest statement
Figures

Similar articles
-
Reinforcement of cancer immunotherapy by adoptive transfer of cblb-deficient CD8+ T cells combined with a DC vaccine.Immunol Cell Biol. 2012 Jan;90(1):130-4. doi: 10.1038/icb.2011.11. Epub 2011 Mar 8. Immunol Cell Biol. 2012. PMID: 21383769
-
Adoptive transfer of siRNA Cblb-silenced CD8+ T lymphocytes augments tumor vaccine efficacy in a B16 melanoma model.PLoS One. 2012;7(9):e44295. doi: 10.1371/journal.pone.0044295. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22962608 Free PMC article.
-
Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation.Mol Immunol. 2009 Dec;47(2-3):164-74. doi: 10.1016/j.molimm.2009.09.026. Epub 2009 Oct 8. Mol Immunol. 2009. PMID: 19818504
-
Releasing the brake: targeting Cbl-b to enhance lymphocyte effector functions.Clin Dev Immunol. 2012;2012:692639. doi: 10.1155/2012/692639. Epub 2012 Apr 10. Clin Dev Immunol. 2012. PMID: 22550535 Free PMC article. Review.
-
Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med. 2012 Feb;271(2):183-92. doi: 10.1111/j.1365-2796.2011.02496.x. Epub 2012 Jan 4. J Intern Med. 2012. PMID: 22126373 Free PMC article. Review.
Cited by
-
The E3 Ubiquitin Ligase c-Cbl Inhibits Microglia-Mediated CNS Inflammation by Regulating PI3K/Akt/NF-κB Pathway.CNS Neurosci Ther. 2016 Aug;22(8):661-9. doi: 10.1111/cns.12557. Epub 2016 May 9. CNS Neurosci Ther. 2016. PMID: 27156691 Free PMC article.
-
E3 ubiquitin ligase Casitas B lineage lymphoma-b and its potential therapeutic implications for immunotherapy.Clin Exp Immunol. 2021 Apr;204(1):14-31. doi: 10.1111/cei.13560. Epub 2021 Jan 10. Clin Exp Immunol. 2021. PMID: 33306199 Free PMC article. Review.
-
Roles of Ubiquitination and Deubiquitination in Regulating Dendritic Cell Maturation and Function.Front Immunol. 2020 Nov 16;11:586613. doi: 10.3389/fimmu.2020.586613. eCollection 2020. Front Immunol. 2020. PMID: 33329564 Free PMC article. Review.
-
Modulation of Immune Cell Functions by the E3 Ligase Cbl-b.Front Oncol. 2015 Mar 11;5:58. doi: 10.3389/fonc.2015.00058. eCollection 2015. Front Oncol. 2015. PMID: 25815272 Free PMC article. Review.
-
Role of Ubiquitin Signaling in Modulating Dendritic Cell Function.Adv Exp Med Biol. 2024;1466:101-111. doi: 10.1007/978-981-97-7288-9_7. Adv Exp Med Biol. 2024. PMID: 39546138 Review.
References
-
- Steinman RM (2012) Decisions about dendritic cells: past, present, and future. Annual Review of Immunology 30: 1–22. - PubMed
-
- Den Brok MHMGM, Nierkens S, Figdor CG, Ruers TJM, Adema GJ (2005) Dendritic cells: tools and targets for antitumor vaccination. Expert Review of Vaccines 4: 699–710. - PubMed
-
- Tjoa BA, Simmons SJ, Bowes VA, Ragde H, Rogers M, et al. (1998) Evaluation of phase I/II clinical trials in prostate cancer with dendritic cells and PSMA peptides. The Prostate 36: 39–44. - PubMed
-
- Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM (2009) Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunology, Immunotherapy 58: 1–14. - PMC - PubMed
-
- Delamarre L, Mellman I (2011) Harnessing dendritic cells for immunotherapy. Seminars in Immunology 23: 2–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

