Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice
- PMID: 23762316
- PMCID: PMC3675204
- DOI: 10.1371/journal.pone.0065201
Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice
Abstract
Background: There are no rigorously confirmed effective medical therapies for calcific aortic stenosis. Hypercholesterolemic Ldlr (-/-) Apob (100/100) mice develop calcific aortic stenosis and valvular cardiomyopathy in old age. Osteoprotegerin (OPG) modulates calcification in bone and blood vessels, but its effect on valve calcification and valve function is not known.
Objectives: To determine the impact of pharmacologic treatment with OPG upon aortic valve calcification and valve function in aortic stenosis-prone hypercholesterolemic Ldlr (-/-) Apob (100/100) mice.
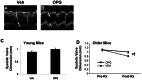
Methods: Young Ldlr (-/-) Apob (100/100) mice (age 2 months) were fed a Western diet and received exogenous OPG or vehicle (N = 12 each) 3 times per week, until age 8 months. After echocardiographic evaluation of valve function, the aortic valve was evaluated histologically. Older Ldlr (-/-) Apob (100/100) mice were fed a Western diet beginning at age 2 months. OPG or vehicle (N = 12 each) was administered from 6 to 12 months of age, followed by echocardiographic evaluation of valve function, followed by histologic evaluation.
Results: In Young Ldlr (-/-) Apob (100/100) mice, OPG significantly attenuated osteogenic transformation in the aortic valve, but did not affect lipid accumulation. In Older Ldlr (-/-) Apob (100/100) mice, OPG attenuated accumulation of the osteoblast-specific matrix protein osteocalcin by ∼80%, and attenuated aortic valve calcification by ∼ 70%. OPG also attenuated impairment of aortic valve function.
Conclusions: OPG attenuates pro-calcific processes in the aortic valve, and protects against impairment of aortic valve function in hypercholesterolemic aortic stenosis-prone Ldlr (-/-) Apob (100/100) mice.
Conflict of interest statement
Figures







Similar articles
-
Pioglitazone attenuates valvular calcification induced by hypercholesterolemia.Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):523-32. doi: 10.1161/ATVBAHA.112.300794. Epub 2013 Jan 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23288158 Free PMC article.
-
Calcific aortic valve stenosis in old hypercholesterolemic mice.Circulation. 2006 Nov 7;114(19):2065-9. doi: 10.1161/CIRCULATIONAHA.106.634139. Epub 2006 Oct 30. Circulation. 2006. PMID: 17075015
-
Increased Calcific Aortic Valve Disease in response to a diabetogenic, procalcific diet in the LDLr-/-ApoB100/100 mouse model.Cardiovasc Pathol. 2018 May-Jun;34:28-37. doi: 10.1016/j.carpath.2018.02.002. Epub 2018 Feb 15. Cardiovasc Pathol. 2018. PMID: 29539583 Free PMC article.
-
Inflammatory and metabolic mechanisms underlying the calcific aortic valve disease.Atherosclerosis. 2018 Oct;277:60-65. doi: 10.1016/j.atherosclerosis.2018.08.029. Epub 2018 Aug 25. Atherosclerosis. 2018. PMID: 30173080 Review.
-
The lipid theory in the pathogenesis of calcific aortic stenosis.Nutr Metab Cardiovasc Dis. 2015 Jun;25(6):519-25. doi: 10.1016/j.numecd.2015.02.001. Epub 2015 Feb 12. Nutr Metab Cardiovasc Dis. 2015. PMID: 25816732 Review.
Cited by
-
Biofunctionalization of decellularized porcine aortic valve with OPG-loaded PCL nanoparticles for anti-calcification.RSC Adv. 2019 Apr 16;9(21):11882-11893. doi: 10.1039/c9ra00408d. eCollection 2019 Apr 12. RSC Adv. 2019. PMID: 35517024 Free PMC article.
-
Uncoupling the Vicious Cycle of Mechanical Stress and Inflammation in Calcific Aortic Valve Disease.Front Cardiovasc Med. 2022 Mar 9;9:783543. doi: 10.3389/fcvm.2022.783543. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35355968 Free PMC article. Review.
-
Role of the RANK/RANKL/OPG and Wnt/β-Catenin Systems in CKD Bone and Cardiovascular Disorders.Calcif Tissue Int. 2021 Apr;108(4):439-451. doi: 10.1007/s00223-020-00803-2. Epub 2021 Feb 13. Calcif Tissue Int. 2021. PMID: 33586001 Review.
-
Pioglitazone attenuates valvular calcification induced by hypercholesterolemia.Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):523-32. doi: 10.1161/ATVBAHA.112.300794. Epub 2013 Jan 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23288158 Free PMC article.
-
The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification.Inflammation. 2021 Apr;44(2):434-449. doi: 10.1007/s10753-020-01357-z. Epub 2020 Nov 20. Inflammation. 2021. PMID: 33215255 Free PMC article. Review.
References
-
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, et al. (2006) Burden of valvular heart diseases: A population-based study. Lancet 368: 1005–1011. - PubMed
-
- Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, et al. (2005) A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 352: 2389–2397. - PubMed
-
- Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, et al. (2008) Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 359: 1343–1356. - PubMed
-
- Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, et al. (2010) Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 121: 306–314. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

