Inverse agonist and pharmacochaperone properties of MK-0524 on the prostanoid DP1 receptor
- PMID: 23762421
- PMCID: PMC3677937
- DOI: 10.1371/journal.pone.0065767
Inverse agonist and pharmacochaperone properties of MK-0524 on the prostanoid DP1 receptor
Abstract
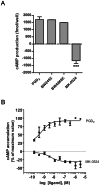
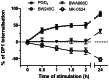
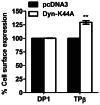
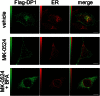
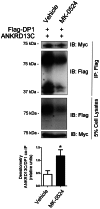
Prostaglandin D₂ (PGD₂) acts through two G protein-coupled receptors (GPCRs), the prostanoid DP receptor and CRTH2 also known as DP1 and DP2, respectively. Several previously characterized GPCR antagonists are now classified as inverse agonists and a number of GPCR ligands are known to display pharmacochaperone activity towards a given receptor. Here, we demonstrate that a DP1 specific antagonist, MK-0524 (also known as laropiprant), decreased basal levels of intracellular cAMP produced by DP1, a Gα(s)-coupled receptor, in HEK293 cells. This reduction in cAMP levels was not altered by pertussis toxin treatment, indicating that MK-0524 did not induce coupling of DP1 to Gα(i/o) proteins and that this ligand is a DP1 inverse agonist. Basal ERK1/2 activation by DP1 was not modulated by MK-0524. Interestingly, treatment of HEK293 cells expressing Flag-tagged DP1 with MK-0524 promoted DP1 cell surface expression time-dependently to reach a maximum increase of 50% compared to control after 24 h. In contrast, PGD₂ induced the internalization of 75% of cell surface DP1 after the same time of stimulation. The increase in DP1 cell surface targeting by MK-0524 was inhibited by Brefeldin A, an inhibitor of transport from the endoplasmic reticulum-Golgi to the plasma membrane. Confocal microscopy confirmed that a large population of DP1 remained trapped intracellularly and co-localized with calnexin, an endoplasmic reticulum marker. Redistribution of DP1 from intracellular compartments to the plasma membrane was observed following treatment with MK-0524 for 24 h. Furthermore, MK-0524 promoted the interaction between DP1 and the ANKRD13C protein, which we showed previously to display chaperone-like effects towards the receptor. We thus report that MK-0524 is an inverse agonist and a pharmacochaperone of DP1. Our findings may have important implications during therapeutic treatments with MK-0524 and for the development of new molecules targeting DP1.
Conflict of interest statement
Figures



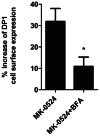
Similar articles
-
The extracellular signal-regulated kinase mitogen-activated protein kinase/ribosomal S6 protein kinase 1 cascade phosphorylates cAMP response element-binding protein to induce MUC5B gene expression via D-prostanoid receptor signaling.J Biol Chem. 2011 Sep 30;286(39):34199-214. doi: 10.1074/jbc.M111.247684. Epub 2011 Aug 10. J Biol Chem. 2011. PMID: 21832046 Free PMC article.
-
Mast cell-derived prostaglandin D2 controls hyaluronan synthesis in human orbital fibroblasts via DP1 activation: implications for thyroid eye disease.J Biol Chem. 2010 May 21;285(21):15794-804. doi: 10.1074/jbc.M109.074534. Epub 2010 Mar 22. J Biol Chem. 2010. PMID: 20308056 Free PMC article.
-
Identification of the interactome of the DP1 receptor for Prostaglandin D2: Regulation of DP1 receptor signaling and trafficking by IQGAP1.Biochim Biophys Acta Gen Subj. 2021 Nov;1865(11):129969. doi: 10.1016/j.bbagen.2021.129969. Epub 2021 Aug 2. Biochim Biophys Acta Gen Subj. 2021. PMID: 34352343
-
Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives.Drugs. 2017 Aug;77(12):1281-1294. doi: 10.1007/s40265-017-0777-2. Drugs. 2017. PMID: 28612233 Free PMC article. Review.
-
Prostaglandin D2 receptor antagonists in allergic disorders: safety, efficacy, and future perspectives.Expert Opin Investig Drugs. 2019 Jan;28(1):73-84. doi: 10.1080/13543784.2019.1555237. Epub 2018 Dec 7. Expert Opin Investig Drugs. 2019. PMID: 30513028 Review.
Cited by
-
Eosinophils Contribute to Intestinal Inflammation via Chemoattractant Receptor-homologous Molecule Expressed on Th2 Cells, CRTH2, in Experimental Crohn's Disease.J Crohns Colitis. 2016 Sep;10(9):1087-95. doi: 10.1093/ecco-jcc/jjw061. Epub 2016 Feb 29. J Crohns Colitis. 2016. PMID: 26928963 Free PMC article.
-
Inverse agonism of SQ 29,548 and Ramatroban on Thromboxane A2 receptor.PLoS One. 2014 Jan 23;9(1):e85937. doi: 10.1371/journal.pone.0085937. eCollection 2014. PLoS One. 2014. PMID: 24465800 Free PMC article.
-
Molecular basis for ligand recognition and receptor activation of the prostaglandin D2 receptor DP1.Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2501902122. doi: 10.1073/pnas.2501902122. Epub 2025 May 29. Proc Natl Acad Sci U S A. 2025. PMID: 40440061
-
Pharmacological chaperoning: a primer on mechanism and pharmacology.Pharmacol Res. 2014 May;83:10-9. doi: 10.1016/j.phrs.2014.01.005. Epub 2014 Feb 14. Pharmacol Res. 2014. PMID: 24530489 Free PMC article. Review.
-
AMG853, A Bispecific Prostaglandin D2 Receptor 1 and 2 Antagonist, Dampens Basophil Activation and Related Lupus-Like Nephritis Activity in Lyn-Deficient Mice.Front Immunol. 2022 Apr 4;13:824686. doi: 10.3389/fimmu.2022.824686. eCollection 2022. Front Immunol. 2022. PMID: 35444641 Free PMC article.
References
-
- Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M (1995) Molecular cloning and characterization of the human prostanoid DP receptor. The Journal of Biological Chemistry 270: 18910–18916. - PubMed
-
- Alving K, Matran R, Lundberg JM (1991) The possible role of prostaglandin D2 in the long-lasting airways vasodilatation induced by allergen in the sensitized pig. Acta Physiologica Scandinavica 143: 93–103. - PubMed
-
- Hardy CC, Robinson C, Tattersfield AE, Holgate ST (1984) The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. The New England Journal of Medicine 311: 209–213. - PubMed
-
- Sturzebecher S, Nieuweboer B, Matthes S, Schillinger E (1989) Effects of PGD2, PGE1, and PGI2-analogues on PGDF-release and aggregation of human gelfiltered platelets. Progress in Clinical and Biological Research 301: 365–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

