Metabolomic profiling unravels DNA adducts in human breast that are formed from peroxidase mediated activation of estrogens to quinone methides
- PMID: 23762435
- PMCID: PMC3675060
- DOI: 10.1371/journal.pone.0065826
Metabolomic profiling unravels DNA adducts in human breast that are formed from peroxidase mediated activation of estrogens to quinone methides
Abstract
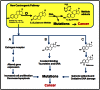
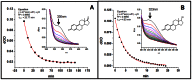
Currently there are three major hypotheses that have been proposed for estrogen induced carcinogenicity, however exact etiology remains unknown. Based on the chemical logic, studies were undertaken to investigate if estrogens could generate quinone methides in an oxidative environment which then could cause DNA damage in humans. In presence of MnO2 estrogens were oxidized to quinone methides. Surprisingly quinone methides were found to be stable with t1/2 of 20.8 and 4.5 min respectively. Incubation of estrogens with lactoperoxidase (LPO) and H2O2 resulted in formation of respective quinone methides (E1(E2)-QM). Subsequent addition of adenine to the assay mixture lead to trapping of E1(E2)-QM, resulting in formation of adenine adducts of estrogens, E1(E2)-9-N-Ade. Targeted ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) based metabolomic analysis of the breast tissue extracts showed the presence of adenine adducts of estrogens, E1(E2)-9-N-Ade, along with other estrogen related metabolites. Identity of E1(E2)-N-Ade in LPO assay extracts and breast tissue extracts were confirmed by comparing them to pure synthesized E1(E2)-9-N-Ade standards. From these results, it is evident that peroxidase enzymes or peroxidase-like activity in human breast tissue could oxidize estrogens to electrophilic and stable quinone methides in a single step that covalently bind to DNA to form adducts. The error prone repair of the damaged DNA can result in mutation of critical genes and subsequently cancer. This article reports evidence for hitherto unknown estrogen metabolic pathway in human breast, catalyzed by peroxidase, which could initiate cancer.
Conflict of interest statement
Figures




References
-
- Gruber C, Tschugguel W, Schneeberger C, Huber J (2002) Production and actions of estrogens. N Engl J Med 346: 340–352. - PubMed
-
- Clemons M, Goss P (2001) Estrogen and the risk of breast cancer. N Engl J Med 344: 276–285. - PubMed
-
- Hein A, Thiel FC, Bayer CM, Fasching PA, Haeberle L, et al. (2013) Hormone replacement therapy and prognosis in ovarian cancer patients. European Journal of Cancer Prevention 22: 52–58. - PubMed
-
- Carruba G (2006) Estrogens and mechanisms of prostate cancer progression. Ann NY Acad Sci 1089: 201–217. - PubMed
-
- Shang Y (2010) Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer 6: 360–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

