Cytochrome P450 3A1 mediates 2,2',4,4'-tetrabromodiphenyl ether-induced reduction of spermatogenesis in adult rats
- PMID: 23762486
- PMCID: PMC3676375
- DOI: 10.1371/journal.pone.0066301
Cytochrome P450 3A1 mediates 2,2',4,4'-tetrabromodiphenyl ether-induced reduction of spermatogenesis in adult rats
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/171e70e3-58e6-4d14-a361-1bc3a9692d46
Abstract
Background: 2,2',4,4'-tetrabromodiphenyl ether (BDE47) is the dominant PBDE congener in humans, wildlife, and the environment. It has been reported to be metabolized by cytochrome P450 (CYP) enzymes. Still, the effects of BDE47 on spermatogenesis failure are attracting an increasing amount of attention. However, it is unclear whether CYP-mediated metabolism contributes to BDE47-induced reproductive toxicity.
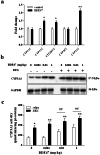
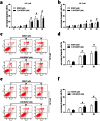
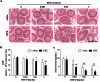
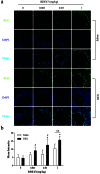
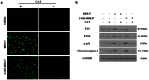
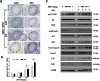
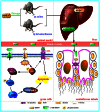
Methodology and principal findings: The role of cytochrome P450 3A1 (CYP3A1) in the formation of oxidative metabolites of BDE47 and its induced spermatogenesis failure was investigated in SD rats. BDE47 significantly increased the expression and activity of CYP3A1 in rat liver, and 3-OH-BDE47, the major oxidative metabolite of BDE47, dose-dependently increased in rat liver, serum, and testis, which was aggravated by dexamethasone (DEX), an inducer of CYP3A1. Additionally, testicular 3-OH-BDE47 and reactive oxygen species (ROS) in seminiferous tubules increased especially when BDE47 was administered in combination with DEX, which was confirmed in GC-1 and GC-2 cells that 3-OH-BDE47 induced more ROS production and cell apoptosis via the upregulation of FAS/FASL, p-p53 and caspase 3. As a result, daily sperm production dose-dependently decreased, consistent with histological observations in giant cells and vacuolar spaces and increase in TUNEL-positive apoptotic germ cells.
Conclusion: CYP3A1-mediated metabolic activation of BDE47 and the active metabolite 3-OH-BDE47 and consequent ROS played an important role in reduction of spermatogenesis by germ cell apoptosis. Our study helps provide new insights into the mechanism of reproductive toxicity of environmental chemicals.
Conflict of interest statement
Figures
Similar articles
-
BDE47 induces rat CYP3A1 by targeting the transcriptional regulation of miR-23b.Sci Rep. 2016 Aug 22;6:31958. doi: 10.1038/srep31958. Sci Rep. 2016. PMID: 27546062 Free PMC article.
-
High-fat diet aggravates 2,2',4,4'-tetrabromodiphenyl ether-inhibited testosterone production via DAX-1 in Leydig cells in rats.Toxicol Appl Pharmacol. 2017 May 15;323:1-8. doi: 10.1016/j.taap.2017.03.010. Epub 2017 Mar 12. Toxicol Appl Pharmacol. 2017. PMID: 28300557
-
Comparative oxidative metabolism of BDE-47 and BDE-99 by rat hepatic microsomes.Toxicol Sci. 2011 Sep;123(1):37-47. doi: 10.1093/toxsci/kfr155. Epub 2011 Jun 14. Toxicol Sci. 2011. PMID: 21673328
-
2,2',4,4'-Tetrabromodiphenyl ether disrupts spermatogenesis, impairs mitochondrial function and induces apoptosis of early leptotene spermatocytes in rats.Reprod Toxicol. 2015 Jan;51:114-24. doi: 10.1016/j.reprotox.2015.01.009. Epub 2015 Feb 2. Reprod Toxicol. 2015. PMID: 25656793
-
Involvement of CYP3A1, 2B1, and 2E1 in C-8 hydroxylation and CYP 1A2 and flavin-containing monooxygenase in N-demethylation of caffeine; identified by using inducer treated rat liver microsomes that are characterized with testosterone metabolic patterns.Chem Biol Interact. 1998 May 1;113(1):1-14. doi: 10.1016/s0009-2797(97)00109-9. Chem Biol Interact. 1998. PMID: 9630843
Cited by
-
Reproductive toxicity of emerging plasticizers, flame retardants, and bisphenols, using culture of the rat fetal testis†.Biol Reprod. 2023 May 10;108(5):837-848. doi: 10.1093/biolre/ioad018. Biol Reprod. 2023. PMID: 36780129 Free PMC article.
-
Differential nanoreprotoxicity of silver nanoparticles in male somatic cells and spermatogonial stem cells.Int J Nanomedicine. 2015 Feb 16;10:1335-57. doi: 10.2147/IJN.S76062. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25733828 Free PMC article.
-
Toxic Effects and Mechanisms of Polybrominated Diphenyl Ethers.Int J Mol Sci. 2023 Aug 30;24(17):13487. doi: 10.3390/ijms241713487. Int J Mol Sci. 2023. PMID: 37686292 Free PMC article. Review.
-
Metabolomics coupled with pathway analysis characterizes metabolic changes in response to BDE-3 induced reproductive toxicity in mice.Sci Rep. 2018 Apr 3;8(1):5423. doi: 10.1038/s41598-018-23484-2. Sci Rep. 2018. PMID: 29615664 Free PMC article.
-
Interaction between β-hexachlorocyclohexane and ADIPOQ genotypes contributes to the risk of type 2 diabetes mellitus in East Chinese adults.Sci Rep. 2016 Nov 24;6:37769. doi: 10.1038/srep37769. Sci Rep. 2016. PMID: 27883041 Free PMC article.
References
-
- Li Q, Yan C, Luo Z, Zhang X (2010) Occurrence and levels of polybrominated diphenyl ethers (PBDEs) in recent sediments and marine organisms from Xiamen offshore areas, China. Mar Pollut Bull 60: 464–469. - PubMed
-
- Mai B, Chen S, Luo X, Chen L, Yang Q, et al. (2005) Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ Sci Technol 39: 3521–3527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

