Egr-1 is a critical regulator of EGF-receptor-mediated expansion of subventricular zone neural stem cells and progenitors during recovery from hypoxia-hypoglycemia
- PMID: 23763269
- PMCID: PMC3786424
- DOI: 10.1042/AN20120032
Egr-1 is a critical regulator of EGF-receptor-mediated expansion of subventricular zone neural stem cells and progenitors during recovery from hypoxia-hypoglycemia
Abstract
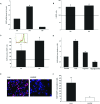
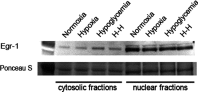
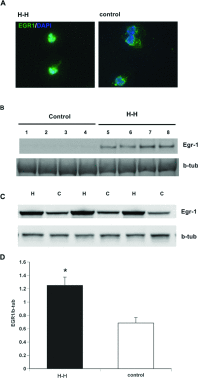
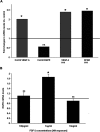
We recently established that the EGF-R (epidermal growth factor receptor) (EGF-R) is an essential regulator of the reactive expansion of SVZ (subventricular zone) NPs (neural precursors) that occurs during recovery from hypoxic-ischemic brain injury. The purpose of the current studies was to identify the conditions and the transcription factor (s) responsible for inducing the EGF-R. Here, we show that the increase in EGF-R expression and the more rapid division of the NPs can be recapitulated in in vitro by exposing SVZ NPs to hypoxia and hypoglycemia simultaneously, but not separately. The EGF-R promoter has binding sites for multiple transcription factors that includes the zinc finger transcription factor, Egr-1. We show that Egr-1 expression increases in NPs, but not astrocytes, following hypoxia and hypoglycemia where it accumulates in the nucleus. To determine whether Egr-1 is necessary for EGF-R expression, we used SiRNAs (small interfering RNA) specific for Egr-1 to decrease Egr-1 expression. Knocking-down Egr-1 decreased basal levels of EGF-R and it abolished the stress-induced increase in EGF-R expression. By contrast, HIF-1 accumulation did not contribute to EGF-R expression and FGF-2 only modestly induced EGF-R. These studies establish a new role for Egr-1 in regulating the expression of the mitogenic EGF-R. They also provide new information into mechanisms that promote NP expansion and provide insights into strategies for amplifying the numbers of stem cells for CNS (central nervous system) regeneration.
Figures

References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Bae S-K, Bae M-H, Ahn M-Y, Son MJ, Lee YM, Bae M-K, Lee O-H, Park BC, Kim K-W. Egr-1 Mediates Transcriptional Activation of IGF-II Gene in Response to Hypoxia. Cancer Res. 1999;59:5989–5994. - PubMed
-
- Bae M-H, Jeong C-H, Kim S-H, Bae M-K, Jeong J-W, Ahn M-Y, Bae S-K, Kim ND, Kim CW, Kim K-R, Kim K-W. Regulation of Egr-1 by association with the proteasome component C8. BBA-MCR. 2002;1592:163–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

