Complexity of trophic factor signaling in experimental autoimmune encephalomyelitis: differential expression of neurotrophic and gliotrophic factors
- PMID: 23763772
- PMCID: PMC3769424
- DOI: 10.1016/j.jneuroim.2013.05.012
Complexity of trophic factor signaling in experimental autoimmune encephalomyelitis: differential expression of neurotrophic and gliotrophic factors
Abstract
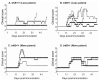
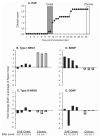
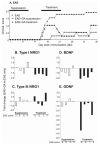
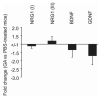
Soluble factors that promote survival and differentiation of glia and neurons during development are likely to play key roles in neurodegeneration and demyelinating diseases such as multiple sclerosis (MS) and have the potential to be important therapeutic targets. We examined the effect of TrkB signaling and the expression patterns of neurotrophic and gliotrophic factors in the mouse brain in MOG-induced experimental allergic encephalomyelitis (EAE). With induction of mild disease, TrkB heterozygous mice were more severely affected compared to their wild type littermates. However, with more potent disease induction, TrkB heterozygotes fared similar to their wild type littermates, suggesting complex modulatory roles for TrkB signaling. One possible explanation for this difference is that the expression patterns of neurotrophic factors correlate with disease severity in individual mice with mild disease, but not in more severe disease. With the less potent induction in C57BL/6 mice, we found that BDNF was consistently increased at EAE onset, while the soluble gliotrophic factor neuregulin (NRG1) was increased only in the chronic phase of the disease. Treatment of these animals with glatiramer acetate (GA) to decrease disease severity resulted in lower levels of both BDNF and NRG1 expression in some mice at 35days after immunization compared to those in untreated EAE mice, but had no direct effect on these factors in the absence of EAE. Our results suggest a complex interplay between neurotrophic and gliotrophic factors in EAE that is dependent on disease stage and severity. While signaling by BDNF through TrkB is protective in mild disease, this effect was not seen in more severe disease. The late induction of NRG1 in the chronic stage of disease could also worsen disease severity through its known ability to activate microglial, inflammatory pathways. While complex, these studies begin to define underlying axoglial trophic activities that are likely involved in both disease pathogenesis and repair.
Keywords: BDNF and TrkB tyrosine kinase receptor; Experimental autoimmune encephalomyelitis; Glatiramer acetate; Multiple sclerosis; Neuregulin1.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Neuregulin1 modulation of experimental autoimmune encephalomyelitis (EAE).J Neuroimmunol. 2018 May 15;318:56-64. doi: 10.1016/j.jneuroim.2018.02.008. Epub 2018 Mar 11. J Neuroimmunol. 2018. PMID: 29534847
-
Restoration of axon conduction and motor deficits by therapeutic treatment with glatiramer acetate.J Neurosci Res. 2014 Dec;92(12):1621-36. doi: 10.1002/jnr.23440. Epub 2014 Jul 3. J Neurosci Res. 2014. PMID: 24989965 Free PMC article.
-
The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice.Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):19045-50. doi: 10.1073/pnas.0509438102. Epub 2005 Dec 19. Proc Natl Acad Sci U S A. 2005. PMID: 16365293 Free PMC article.
-
Brain-derived neurotrophic factor and TrkB receptor in experimental autoimmune encephalomyelitis and multiple sclerosis.J Neurol Sci. 2009 Dec 15;287(1-2):17-26. doi: 10.1016/j.jns.2009.08.057. Epub 2009 Sep 16. J Neurol Sci. 2009. PMID: 19758606 Review.
-
BDNF: An Oncogene or Tumor Suppressor?Anticancer Res. 2017 Aug;37(8):3983-3990. doi: 10.21873/anticanres.11783. Anticancer Res. 2017. PMID: 28739680 Review.
Cited by
-
Molecular Effects of FDA-Approved Multiple Sclerosis Drugs on Glial Cells and Neurons of the Central Nervous System.Int J Mol Sci. 2020 Jun 13;21(12):4229. doi: 10.3390/ijms21124229. Int J Mol Sci. 2020. PMID: 32545828 Free PMC article. Review.
-
Side effect profile similarities shared between antidepressants and immune-modulators reveal potential novel targets for treating major depressive disorders.BMC Pharmacol Toxicol. 2016 Oct 21;17(1):47. doi: 10.1186/s40360-016-0090-9. BMC Pharmacol Toxicol. 2016. PMID: 27765060 Free PMC article.
-
Estrogen receptor (ER) β expression in oligodendrocytes is required for attenuation of clinical disease by an ERβ ligand.Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):19125-30. doi: 10.1073/pnas.1311763110. Epub 2013 Nov 4. Proc Natl Acad Sci U S A. 2013. PMID: 24191028 Free PMC article.
-
Disease Modifying Potential of Glatiramer Acetate in Huntington's Disease.J Huntingtons Dis. 2014;3(3):311-6. doi: 10.3233/JHD-140110. J Huntingtons Dis. 2014. PMID: 25300334 Free PMC article.
References
-
- Aharoni R, Saada R, Eilam R, Hayardeny L, Sela M, Arnon R. Oral treatment with laquinimod augments regulatory T-cells and brain-derived neurotrophic factor expression and reduces injury in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2012;251:14–24. - PubMed
-
- Bredesen DE, Rabizadeh S. p75NTR and apoptosis: Trk-dependent and Trk-independent effects. Trends Neurosci. 1997;20:287–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

