SYK inhibition modulates distinct PI3K/AKT- dependent survival pathways and cholesterol biosynthesis in diffuse large B cell lymphomas
- PMID: 23764004
- PMCID: PMC3700321
- DOI: 10.1016/j.ccr.2013.05.002
SYK inhibition modulates distinct PI3K/AKT- dependent survival pathways and cholesterol biosynthesis in diffuse large B cell lymphomas
Abstract
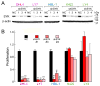
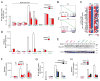
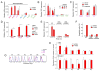
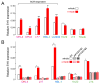
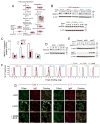
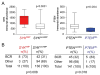
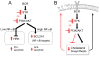
B cell receptor (BCR) signaling pathway components represent promising treatment targets in diffuse large B cell lymphoma (DLBCL) and additional B cell tumors. BCR signaling activates spleen tyrosine kinase (SYK) and downstream pathways including PI3K/AKT and NF-κB. In previous studies, chemical SYK blockade selectively decreased BCR signaling and induced apoptosis of BCR-dependent DLBCLs. Herein, we characterize distinct SYK/PI3K-dependent survival pathways in DLBCLs with high or low baseline NF-κB activity including selective repression of the pro-apoptotic HRK protein in NF-κB-low tumors. We also define SYK/PI3K-dependent cholesterol biosynthesis as a feed-forward mechanism of maintaining the integrity of BCRs in lipid rafts in DLBCLs with low or high NF-κB. In addition, SYK amplification and PTEN deletion are identified as selective genetic alterations in primary "BCR"-type DLBCLs.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Bengoechea-Alonso M, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Current Opinion in Cell Biology. 2007;19:215–222. - PubMed
-
- Chen L, Juszczynski P, Takeyama K, Aguiar RC, Shipp MA. Protein tyrosine phosphatase receptor-type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell-receptor signaling, and cellular proliferation. Blood. 2006;108:3428–3433. - PubMed
-
- Cheng S, Coffey G, Zhang XH, Shaknovich R, Song Z, Lo P, Pandey A, Melnick AM, Somma U, Wang L. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood. 2011;118:6342–6352. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

