Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death
- PMID: 23764021
- PMCID: PMC3686663
- DOI: 10.1186/1471-2407-13-285
Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death
Abstract
Background: Recent research has revealed that targeting mitochondrial bioenergetic metabolism is a promising chemotherapeutic strategy. Key to successful implementation of this chemotherapeutic strategy is the use of new and improved mitochondria-targeted cationic agents that selectively inhibit energy metabolism in breast cancer cells, while exerting little or no long-term cytotoxic effect in normal cells.
Methods: In this study, we investigated the cytotoxicity and alterations in bioenergetic metabolism induced by mitochondria-targeted vitamin E analog (Mito-chromanol, Mito-ChM) and its acetylated ester analog (Mito-ChMAc). Assays of cell death, colony formation, mitochondrial bioenergetic function, intracellular ATP levels, intracellular and tissue concentrations of tested compounds, and in vivo tumor growth were performed.
Results: Both Mito-ChM and Mito-ChMAc selectively depleted intracellular ATP and caused prolonged inhibition of ATP-linked oxygen consumption rate in breast cancer cells, but not in non-cancerous cells. These effects were significantly augmented by inhibition of glycolysis. Mito-ChM and Mito-ChMAc exhibited anti-proliferative effects and cytotoxicity in several breast cancer cells with different genetic background. Furthermore, Mito-ChM selectively accumulated in tumor tissue and inhibited tumor growth in a xenograft model of human breast cancer.
Conclusions: We conclude that mitochondria-targeted small molecular weight chromanols exhibit selective anti-proliferative effects and cytotoxicity in multiple breast cancer cells, and that esterification of the hydroxyl group in mito-chromanols is not a critical requirement for its anti-proliferative and cytotoxic effect.
Figures



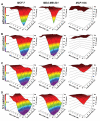
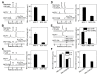

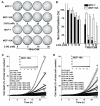
References
-
- Beckham TH, Lu P, Jones EE, Marrison T, Lewis CS, Cheng JC, Ramshesh VK, Beeson G, Beeson CC, Drake RR, Bielawska A, Bielawski J, Szulc ZM, Ogretmen B, Norris JS, Liu X. LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. J Pharmacol Exp Ther. 2013;344:167–178. doi: 10.1124/jpet.112.199216. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

