Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations
- PMID: 23764283
- PMCID: PMC3782391
- DOI: 10.1016/j.neuron.2013.03.025
Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations
Erratum in
- Neuron. 2013 Sep 18;79(6):1257
Abstract
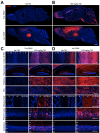
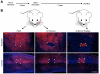
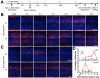
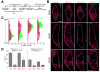
Targeting genetically encoded tools for neural circuit dissection to relevant cellular populations is a major challenge in neurobiology. We developed an approach, targeted recombination in active populations (TRAP), to obtain genetic access to neurons that were activated by defined stimuli. This method utilizes mice in which the tamoxifen-dependent recombinase CreER(T2) is expressed in an activity-dependent manner from the loci of the immediate early genes Arc and Fos. Active cells that express CreER(T2) can only undergo recombination when tamoxifen is present, allowing genetic access to neurons that are active during a time window of less than 12 hr. We show that TRAP can provide selective access to neurons activated by specific somatosensory, visual, and auditory stimuli and by experience in a novel environment. When combined with tools for labeling, tracing, recording, and manipulating neurons, TRAP offers a powerful approach for understanding how the brain processes information and generates behavior.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Amir S, Robinson B. Fos expression in rat visual cortex induced by ocular input of ultraviolet light. Brain Res. 1996;716:213–218. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J. Neurophysiol. 1992;68:1345–1358. - PubMed
-
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of arc rna in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

