Aging in the cerebellum and hippocampus and associated behaviors over the adult life span of CB6F1 mice
- PMID: 23764510
- PMCID: PMC3755498
- DOI: 10.1016/j.neuroscience.2013.06.002
Aging in the cerebellum and hippocampus and associated behaviors over the adult life span of CB6F1 mice
Abstract
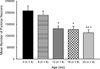
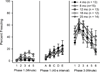
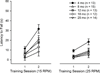
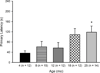
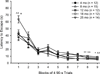
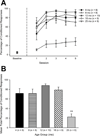
In the present study we examined the effects of normal aging in the hippocampus and cerebellum, as well as behaviors associated with these substrates. A total of 67 CB6F1 hybrid mice were tested at one of five ages (4, 8, 12, 18 or 25 months) on the context pre-exposure facilitation effect (CPFE) modification of fear conditioning, rotorod, Barnes maze, acoustic startle, Morris water maze (MWM) and 500-ms trace eyeblink classical conditioning (EBCC). Behavioral tasks were chosen to increase the ability to detect age-related changes in learning, as trace EBCC is considered a more difficult paradigm (compared to delay EBCC) and the CPFE has been found to be more sensitive to hippocampus insults than standard contextual fear conditioning. To assess the effects of age on the brain, hippocampus volume was calculated and unbiased stereology was used to estimate the number of Purkinje neurons in the cerebellar cortex. A significant, age-related loss of Purkinje neurons was found-beginning at 12 months of age-and hippocampus volume remained stable over the adult life span. Age-related impairment was found, beginning at 12-18 months in the rotorod, and mice with fewer Purkinje neurons showed greater impairment in this task. CB6F1 mice retained auditory acuity across the life span and mice aged 25 months showed significant age-related impairment in the EBCC task; however, deficits were not associated with the loss of Purkinje neurons. Although the CPFE task is considered more sensitive to hippocampus insult, no age-related impairment was found. Spatial memory retention was impaired in the Barnes maze at 25 months, but no significant deficits were seen in the MWM. These results support the finding of differential aging in the hippocampus and cerebellum.
Keywords: ANOVA; CB6F1; CPFE; CRs; EBCC; EMG; MWM; Morris water maze; SD; SMART; Spontaneous Motor Activity Recording and Tracking; age sensitivity; aging; analysis of variance; cerebellum; conditioned responses; context pre-exposure facilitation effect; electromyography; eyeblink classical conditioning; hippocampus; learning; standard deviation.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures

References
-
- Anderson B, Gunderson H, Pakkenberg B. Aging of the human cerebellum: A stereological study. J Comp Neurol. 2003;466:356–365. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96(9):5280–5285. - PMC - PubMed
-
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76(3):447–461. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

