Identification and optimization of small-molecule agonists of the human relaxin hormone receptor RXFP1
- PMID: 23764525
- PMCID: PMC4915074
- DOI: 10.1038/ncomms2953
Identification and optimization of small-molecule agonists of the human relaxin hormone receptor RXFP1
Abstract
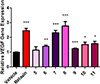
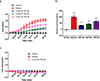
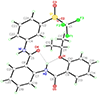
The anti-fibrotic, vasodilatory and pro-angiogenic therapeutic properties of recombinant relaxin peptide hormone have been investigated in several diseases, and recent clinical trial data has shown benefit in treating acute heart failure. However, the remodelling capacity of these peptide hormones is difficult to study in chronic settings because of their short half-life and the need for intravenous administration. Here we present the first small-molecule series of human relaxin/insulin-like family peptide receptor 1 agonists. These molecules display similar efficacy as the natural hormone in several functional assays. Mutagenesis studies indicate that the small molecules activate relaxin receptor through an allosteric site. These compounds have excellent physical and in vivo pharmacokinetic properties to support further investigation of relaxin biology and animal efficacy studies of the therapeutic benefits of relaxin/insulin-like family peptide receptor 1 activation.
Figures
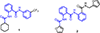


Similar articles
-
Structural Insights into the Activation of Human Relaxin Family Peptide Receptor 1 by Small-Molecule Agonists.Biochemistry. 2016 Mar 29;55(12):1772-83. doi: 10.1021/acs.biochem.5b01195. Epub 2016 Mar 4. Biochemistry. 2016. PMID: 26866459 Free PMC article.
-
Identification of small-molecule agonists of human relaxin family receptor 1 (RXFP1) by using a homogenous cell-based cAMP assay.J Biomol Screen. 2013 Jul;18(6):670-7. doi: 10.1177/1087057112469406. Epub 2012 Dec 4. J Biomol Screen. 2013. PMID: 23212924 Free PMC article.
-
Optimization of the first small-molecule relaxin/insulin-like family peptide receptor (RXFP1) agonists: Activation results in an antifibrotic gene expression profile.Eur J Med Chem. 2018 Aug 5;156:79-92. doi: 10.1016/j.ejmech.2018.06.008. Epub 2018 Jun 7. Eur J Med Chem. 2018. PMID: 30006176 Free PMC article.
-
Synthetic non-peptide low molecular weight agonists of the relaxin receptor 1.Br J Pharmacol. 2017 May;174(10):977-989. doi: 10.1111/bph.13656. Epub 2016 Nov 30. Br J Pharmacol. 2017. PMID: 27771940 Free PMC article. Review.
-
Single chain peptide agonists of relaxin receptors.Mol Cell Endocrinol. 2019 May 1;487:34-39. doi: 10.1016/j.mce.2019.01.008. Epub 2019 Jan 11. Mol Cell Endocrinol. 2019. PMID: 30641102 Review.
Cited by
-
Discovery of small molecule agonists of the Relaxin Family Peptide Receptor 2.Commun Biol. 2022 Nov 4;5(1):1183. doi: 10.1038/s42003-022-04143-9. Commun Biol. 2022. PMID: 36333465 Free PMC article.
-
The complex binding mode of the peptide hormone H2 relaxin to its receptor RXFP1.Nat Commun. 2016 Apr 18;7:11344. doi: 10.1038/ncomms11344. Nat Commun. 2016. PMID: 27088579 Free PMC article.
-
ML290 is a biased allosteric agonist at the relaxin receptor RXFP1.Sci Rep. 2017 Jun 7;7(1):2968. doi: 10.1038/s41598-017-02916-5. Sci Rep. 2017. PMID: 28592882 Free PMC article.
-
Structural Insights into the Activation of Human Relaxin Family Peptide Receptor 1 by Small-Molecule Agonists.Biochemistry. 2016 Mar 29;55(12):1772-83. doi: 10.1021/acs.biochem.5b01195. Epub 2016 Mar 4. Biochemistry. 2016. PMID: 26866459 Free PMC article.
-
Allosteric and biased g protein-coupled receptor signaling regulation: potentials for new therapeutics.Front Endocrinol (Lausanne). 2014 May 8;5:68. doi: 10.3389/fendo.2014.00068. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 24847311 Free PMC article. Review.
References
-
- Hisaw FL. Experimental relaxation of the pubic ligament of the guinea pig. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 1926;23:661–663.
-
- Fevold HL, Hisaw FL, Meyer RK. The relaxative hormone of the corpus luteum. Its purification and concnetration. J. Am. Chem. Soc. 1930;52:3340–3348.
-
- Baylis C. Relaxin may be the "elusive" renal vasodilatory agent of normal pregnancy. Am. J. Kidney Dis. 1999;34:1142–1144. - PubMed
-
- Jeyabalan A, Shroff SG, Novak J, Conrad KP. The vascular actions of relaxin. Adv. Exp. Med. Biol. 2007;612:65–87. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

