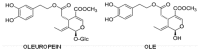Protein folding and aggregation into amyloid: the interference by natural phenolic compounds
- PMID: 23765219
- PMCID: PMC3709793
- DOI: 10.3390/ijms140612411
Protein folding and aggregation into amyloid: the interference by natural phenolic compounds
Abstract
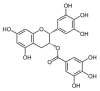
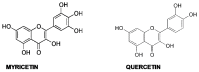
Amyloid aggregation is a hallmark of several degenerative diseases affecting the brain or peripheral tissues, whose intermediates (oligomers, protofibrils) and final mature fibrils display different toxicity. Consequently, compounds counteracting amyloid aggregation have been investigated for their ability (i) to stabilize toxic amyloid precursors; (ii) to prevent the growth of toxic oligomers or speed that of fibrils; (iii) to inhibit fibril growth and deposition; (iv) to disassemble preformed fibrils; and (v) to favor amyloid clearance. Natural phenols, a wide panel of plant molecules, are one of the most actively investigated categories of potential amyloid inhibitors. They are considered responsible for the beneficial effects of several traditional diets being present in green tea, extra virgin olive oil, red wine, spices, berries and aromatic herbs. Accordingly, it has been proposed that some natural phenols could be exploited to prevent and to treat amyloid diseases, and recent studies have provided significant information on their ability to inhibit peptide/protein aggregation in various ways and to stimulate cell defenses, leading to identify shared or specific mechanisms. In the first part of this review, we will overview the significance and mechanisms of amyloid aggregation and aggregate toxicity; then, we will summarize the recent achievements on protection against amyloid diseases by many natural phenols.
Figures
References
-
- Levinthal C. Are there pathways for protein folding? J. Chem. Phys. 1968;85:44–45.
-
- Radford S.E., Dobson C.M. From computer simulations to human disease: Emerging themes in protein folding. Cell. 1999;97:291–298. - PubMed
-
- Stefani M., Dobson C.M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003;81:678–699. - PubMed
-
- Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. - PubMed
-
- Thomas P.J., Qu B.-H., Pedersen P.L. Defective protein folding as a basis of human disease. Trends Biochem. Sci. 1995;20:456–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources