Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation
- PMID: 23765266
- PMCID: PMC3751230
- DOI: 10.1007/s00425-013-1907-z
Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation
Abstract
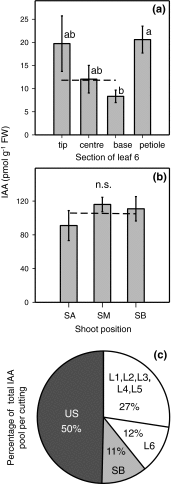
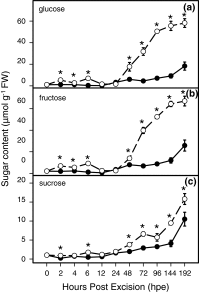
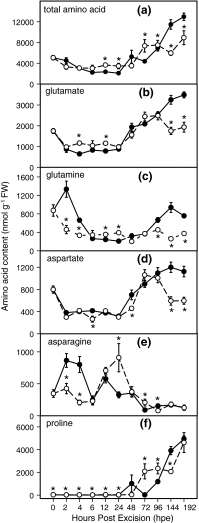
To determine the contribution of polar auxin transport (PAT) to auxin accumulation and to adventitious root (AR) formation in the stem base of Petunia hybrida shoot tip cuttings, the level of indole-3-acetic acid (IAA) was monitored in non-treated cuttings and cuttings treated with the auxin transport blocker naphthylphthalamic acid (NPA) and was complemented with precise anatomical studies. The temporal course of carbohydrates, amino acids and activities of controlling enzymes was also investigated. Analysis of initial spatial IAA distribution in the cuttings revealed that approximately 40 and 10 % of the total IAA pool was present in the leaves and the stem base as rooting zone, respectively. A negative correlation existed between leaf size and IAA concentration. After excision of cuttings, IAA showed an early increase in the stem base with two peaks at 2 and 24 h post excision and, thereafter, a decline to low levels. This was mirrored by the expression pattern of the auxin-responsive GH3 gene. NPA treatment completely suppressed the 24-h peak of IAA and severely inhibited root formation. It also reduced activities of cell wall and vacuolar invertases in the early phase of AR formation and inhibited the rise of activities of glucose-6-phosphate dehydrogenase and phosphofructokinase during later stages. We propose a model in which spontaneous AR formation in Petunia cuttings is dependent on PAT and on the resulting 24-h peak of IAA in the rooting zone, where it induces early cellular events and also stimulates sink establishment. Subsequent root development stimulates glycolysis and the pentose phosphate pathway.
Figures






References
-
- Agulló-Antón MA, Sánchez-Bravo J, Acosta M, Druege U. Auxins or sugars: what makes the difference in the adventitious rooting of stored carnation cuttings? J Plant Growth Regul. 2011;30:100–113. doi: 10.1007/s00344-010-9174-8. - DOI
-
- Ahkami AH, Lischewski S, Haensch KT, Porfirova S, Hofmann J, Rolletschek H, Melzer M, Franken P, Hause B, Druege U, Hajirezaei MR. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol. 2009;181:613–625. doi: 10.1111/j.1469-8137.2008.02704.x. - DOI - PubMed
-
- Albacate A, Grosskinsky DK, Roitsch TH. Trick and treat: a review on the function and regulation of plant invertases in the abiotic stress response. Phyton. 2011;50:181–204.
-
- Altman A, Wareing PF. Effect of IAA on sugar accumulation and basipetal transport of C14-labeled assimilates in relation to root formation in Phaseolus vulgaris cuttings. Physiol Plant. 1975;33:32–38. doi: 10.1111/j.1399-3054.1975.tb03760.x. - DOI
-
- Baadsmand S, Andersen AS. Transport and accumulation of indole-3-acetic acid in pea cuttings under two levels of irradiance. Physiol Plant. 1984;61:107–113. doi: 10.1111/j.1399-3054.1984.tb06108.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

