Toward RNAi therapy for the polyglutamine disease Machado-Joseph disease
- PMID: 23765441
- PMCID: PMC3808129
- DOI: 10.1038/mt.2013.144
Toward RNAi therapy for the polyglutamine disease Machado-Joseph disease
Abstract
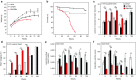
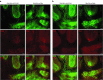
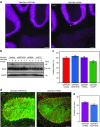
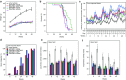
Machado-Joseph disease (MJD) is a dominantly inherited ataxia caused by a polyglutamine-coding expansion in the ATXN3 gene. Suppressing expression of the toxic gene product represents a promising approach to therapy for MJD and other polyglutamine diseases. We performed an extended therapeutic trial of RNA interference (RNAi) targeting ATXN3 in a mouse model expressing the full human disease gene and recapitulating key disease features. Adeno-associated virus (AAV) encoding a microRNA (miRNA)-like molecule, miRATXN3, was delivered bilaterally into the cerebellum of 6- to 8-week-old MJD mice, which were then followed up to end-stage disease to assess the safety and efficacy of anti-ATXN3 RNAi. Despite effective, lifelong suppression of ATXN3 in the cerebellum and the apparent safety of miRATXN3, motor impairment was not ameliorated in treated MJD mice and survival was not prolonged. These results with an otherwise effective RNAi agent suggest that targeting a large extent of the cerebellum alone may not be sufficient for effective human therapy. Artificial miRNAs or other nucleotide-based suppression strategies targeting ATXN3 more widely in the brain should be considered in future preclinical tests.
Figures



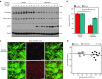
References
-
- Lima M, Costa MC, Montiel R, Ferro A, Santos C, Silva C, et al. Population genetics of wild-type CAG repeats in the Machado-Joseph disease gene in Portugal. Hum Hered. 2005;60:156–163. - PubMed
-
- Maciel P, Costa MC, Ferro A, Rousseau M, Santos CS, Gaspar C, et al. Improvement in the molecular diagnosis of Machado-Joseph disease. Arch Neurol. 2001;58:1821–1827. - PubMed
-
- Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978;28:703–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

