Repetitively dosed docetaxel and ¹⁵³samarium-EDTMP as an antitumor strategy for metastatic castration-resistant prostate cancer
- PMID: 23765638
- PMCID: PMC3775970
- DOI: 10.1002/cncr.28103
Repetitively dosed docetaxel and ¹⁵³samarium-EDTMP as an antitumor strategy for metastatic castration-resistant prostate cancer
Abstract
Background: β-emitting bone-seeking radiopharmaceuticals have historically been administered for pain palliation whereas docetaxel prolongs life in patients with metastatic castration-resistant prostate cancer (mCRPC). In combination, these agents simultaneously target the bone stroma and cancer cell to optimize antitumor effects. The toxicity and efficacy when each agent is combined at full, recommended doses, in a repetitive fashion is not well established.
Methods: Patients with progressive mCRPC and ≥ 3 bone lesions received (153) Sm-EDTMP (samarium-153 ethylene diamine tetramethylene phosphonate) at a dose of 1.0 mCi/kg every 9 weeks and docetaxel at a dose of 75 mg/m(2) every 3 weeks. In the absence of unacceptable toxicity, patients were allowed to continue additional cycles, defined by 9 weeks of treatment, until intolerance or biochemical/radiographic disease progression.
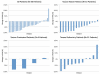
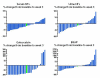
Results: Of the 30 patients treated, approximately 50% were considered to be taxane-naive, 36.7% were taxane-refractory, and 13.3% had previously been exposed to taxanes but were not considered refractory. Patients received on average 2.5 cycles of treatment (6.5 doses of docetaxel and 2.5 doses of (153) Sm-EDTMP). Twelve patients (40%) demonstrated a decline in their prostate-specific antigen level of ≥ 50%. The median progression-free survival (biochemical or radiographic) was 7.0 months and the overall survival was 14.3 months. Nine patients (30%) did not recover platelet counts >100 K/mm(3) after a median of 3 cycles to allow for additional treatment, with 4 patients experiencing prolonged thrombocytopenia. The most common reasons for trial discontinuation were progressive disease and hematologic toxicity.
Conclusions: The results of the current study indicate that (153) Sm-EDTMP can be safely combined with docetaxel at full doses on an ongoing basis in patients with mCRPC. Although thrombocytopenia limited therapy for some patients, preliminary efficacy supports the strategy of combining a radiopharmaceutical with chemotherapy, which is an appealing strategy given the anticipated availability of α emitters that can prolong survival.
Keywords: 153Sm-EDTMP (samarium-153 ethylene diamine tetramethylene phosphonate); chemotherapy; docetaxel; prostate cancer; radiopharmaceutical.
Copyright © 2013 American Cancer Society.
Figures




References
-
- Morris MJ, Pandit-Taskar N, Stephenson RD, et al. Phase I/II study of docetaxel and 153Sm for castrate metastatic prostate cancer (CMPC): Summary of dose-escalation cohorts and first report on the expansion cohort. J Clin Oncol. 2009;27:15s. (suppl; abstr 5057)
-
- National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v3.0. 2006 http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/....
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. - PubMed
-
- Parker C, Heinrich D, Sullivan JM, et al. Overall survival benefit of Radium 223 Chloride (Alpharadin) in the treatment of patients with symptomatic bone metastases in castrate resistant prostate cancer: a Phase III randomized trial. European Multidisciplinary Cancer Congress; Stockholm: 2011.
-
- Fizazi K, Beuzeboc P, Lumbroso J, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27:2429–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

