Brief report: VGLL4 is a novel regulator of survival in human embryonic stem cells
- PMID: 23765749
- PMCID: PMC4617635
- DOI: 10.1002/stem.1445
Brief report: VGLL4 is a novel regulator of survival in human embryonic stem cells
Abstract
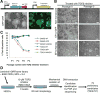
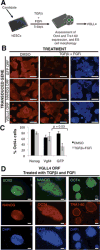
Human embryonic stem cells (hESCs) are maintained in a self-renewing state by an interconnected network of mechanisms that sustain pluripotency, promote proliferation and survival, and prevent differentiation. We sought to find novel genes that could contribute to one or more of these processes using a gain-of-function screen of a large collection of human open reading frames. We identified Vestigial-like 4 (VGLL4), a cotranscriptional regulator with no previously described function in hESCs, as a positive regulator of survival in hESCs. Specifically, VGLL4 overexpression in hESCs significantly decreases cell death in response to dissociation stress. Additionally, VGLL4 overexpression enhances hESC colony formation from single cells. These effects may be attributable, in part, to a decreased activity of initiator and effector caspases observed in the context of VGLL4 overexpression. Additionally, we show an interaction between VGLL4 and the Rho/Rock pathway, previously implicated in hESC survival. This study introduces a novel gain-of-function approach for studying hESC maintenance and presents VGLL4 as a previously undescribed regulator of this process. Stem Cells 2013;31:2833-2841.
Keywords: Apoptosis; Pluripotent stem cells; Rho-associated kinases; VGLL4 protein.
© AlphaMed Press.
Figures

References
-
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007 Jun;25:681–6. - PubMed
-
- Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, Ishizaki T, Suemori H, Narumiya S, Niwa H, Sasai Y. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010 Aug 6;7:225–39. - PubMed
-
- Ohgushi M, Sasai Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends in Cell Biology. 2011;21:274–282. - PubMed
-
- Murphy GJ, Mostoslavsky G, Kotton DN, Mulligan RC. Exogenous control of mammalian gene expression via modulation of translational termination. Nat Med. 2006;12:1093–1099. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

